��2010?��������ģ����������ƣ�Na
2S
2O
3�������������ƺ����ͨ�����Ϸ�Ӧ�Ƶã���֪��Na
2S
2O
3��������Һ�в����ȶ����ڣ�
��1��ij�о�С��������Ʊ�Na
2S
2O
3?5H
2O��װ�úͲ��ֲ����������£�
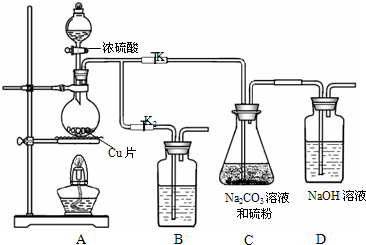
��K
1���ر�K
2����Բ����ƿ�м�������Ũ���ᣬ���ȣ�
��C�еĻ��Һ��������������Ӧһ��ʱ�����۵������٣���C����Һ��pH�ӽ�7ʱ��ֹͣC�еķ�Ӧ��ֹͣ���ȣ�
����C�еĻ��Һ��
��������Һ����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ���ɣ��õ���Ʒ��
�٢��У�Բ����ƿ�з�����Ӧ�Ļ�ѧ����ʽ��
Cu+2H
2SO
4��Ũ��
CuSO
4+SO
2��+2H
2O
Cu+2H
2SO
4��Ũ��
CuSO
4+SO
2��+2H
2O
��
�ڢ��У�����C����Һ��pH�ӽ�7ʱ��ֹͣC�еķ�Ӧ����ԭ����
Na2S2O3��������Һ�в����ȶ�����
Na2S2O3��������Һ�в����ȶ�����
��
��ֹͣC�еķ�Ӧ���IJ�����
��K2���ر�K1
��K2���ر�K1
��
�ۢ��У������ˡ��õ��IJ��������ǣ����������ƣ�
©�������������ձ�
©�������������ձ�
��
��װ��B��ʢ�ŵ��Լ��ǣ��ѧʽ��
NaOH
NaOH
��Һ����������
��C�еķ�Ӧֹͣ������A�в����Ķ���SO2����ֹ������Ⱦ
��C�еķ�Ӧֹͣ������A�в����Ķ���SO2����ֹ������Ⱦ
��
��2�����ݷ�Ӧ2S
2O
32-+I
2=S
4O
62-+2I
-������I
2�ı���Һ�ⶨ��Ʒ�Ĵ��ȣ�ȡ5.5g ��Ʒ�����Ƴ�100mL��Һ��ȡ10mL��Һ���Ե�����ҺΪָʾ������Ũ��Ϊ0.050mol/L I
2�ı���Һ���еζ���������ݼ�¼���±���ʾ��
| �� �� |
1 |
2 |
3 |
4 |
| ��Һ�����/mL |
10.00 |
10.00 |
10.00 |
10.00 |
| ����I2����Һ�����/mL |
19.99 |
19.98 |
17.13 |
20.03 |
���жϴﵽ�ζ��յ��������
�������һ��I2����Һ����Һ�������Ұ��������ɫ���ı�
�������һ��I2����Һ����Һ�������Ұ��������ɫ���ı�
��
��Na
2S
2O
3?5H
2O�ڲ�Ʒ�е����������ǣ�����������1λС����
90.2%
90.2%
����Na
2S
2O
3?5H
2O��ʽ��Ϊ248��




