
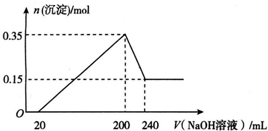
 ½«Ņ»¶ØÖŹĮæµÄĆ¾ŗĶĀĮ»ģŗĻĪļĶ¶Čė200 mLĻ”ĮņĖįÖŠ£¬¹ĢĢåČ«²æČܽāŗó£¬ĻņĖłµĆČÜŅŗÖŠ¼ÓČėNaOHČÜŅŗ£¬Éś³É³ĮµķµÄĪļÖŹµÄĮæÓė¼ÓČėµÄNaOHČÜŅŗĢå»żµÄ±ä»Æ¹ŲĻµČēĶ¼ĖłŹ¾£®ŌņĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ£Ø””””£©
½«Ņ»¶ØÖŹĮæµÄĆ¾ŗĶĀĮ»ģŗĻĪļĶ¶Čė200 mLĻ”ĮņĖįÖŠ£¬¹ĢĢåČ«²æČܽāŗó£¬ĻņĖłµĆČÜŅŗÖŠ¼ÓČėNaOHČÜŅŗ£¬Éś³É³ĮµķµÄĪļÖŹµÄĮæÓė¼ÓČėµÄNaOHČÜŅŗĢå»żµÄ±ä»Æ¹ŲĻµČēĶ¼ĖłŹ¾£®ŌņĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ£Ø””””£©| A£® | Ć¾ŗĶĀĮµÄ×ÜÖŹĮæĪŖ9 g | |
| B£® | ×ī³õ20 mL NaOHČÜŅŗÓĆÓŚÖŠŗĶ¹żĮæµÄĻ”ĮņĖį | |
| C£® | ĒāŃõ»ÆÄĘČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ5 mol•L-1 | |
| D£® | Éś³ÉµÄĒāĘųŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ11.2 L |
·ÖĪö ÓÉĶ¼ĻóæÉÖŖ£¬“ÓæŖŹ¼ÖĮ¼ÓČėNaOHČÜŅŗ20mL£¬Ć»ÓŠ³ĮµķÉś³É£¬ĖµĆ÷ŌČÜŅŗÖŠĮņĖįČܽāMg”¢AlŗóĮņĖįÓŠŹ£Óą£¬“ĖŹ±·¢ÉśµÄ·“Ó¦ĪŖ£ŗH2SO4+2NaOH=Na2SO4+2H2O£®µ±V£ØNaOHČÜŅŗ£©=200mLŹ±£¬³ĮµķĮæ×ī“󣬓ĖŹ±ĪŖMg£ØOH£©2ŗĶAl£ØOH£©3£¬¶žÕßĪļÖŹµÄĮæÖ®ŗĶĪŖ0.35mol£¬ČÜŅŗÖŠČÜÖŹĪŖNa2SO4£¬øł¾ŻÄĘŌŖĖŲŹŲŗćæÉÖŖ“ĖŹ±n£ØNa2SO4£©µČÓŚ200mLĒāŃõ»ÆÄĘČÜŅŗÖŠŗ¬ÓŠµÄn£ØNaOH£©µÄ$\frac{1}{2}$±¶£®“Ó200mLµ½240mL£¬NaOHČܽāAl£ØOH£©3£ŗNaOH+Al£ØOH£©3=NaAlO2+2H2O£¬µ±V£ØNaOHČÜŅŗ£©=240mLŹ±£¬³Įµķ²»ŌŁ¼õÉŁ£¬“ĖŹ±Č«²æĪŖMg£ØOH£©2£¬ĪļÖŹµÄĮæĪŖ0.15mol£¬ĖłŅŌ³ĮµķĮæ×ī“ó£¬Mg£ØOH£©2ĪŖ0.15mol£¬Al£ØOH£©3ĪŖ0.35mol-0.15mol=0.2mol£¬ÓÉÓŚ“Ó200mLµ½240mL£¬NaOHČܽāAl£ØOH£©3£ŗNaOH+Al£ØOH£©3=NaAlO2+2H2O£¬ĖłŅŌøĆ½×¶ĪĻūŗÄn£ØNaOH£©=n[Al£ØOH£©3]=0.2mol£¬ĒāŃõ»ÆÄʵÄÅضČĪŖ$\frac{0.2mol}{0.24L-0.20L}$=5mol/L£¬ŅŌ“ĖĄ“½ā“š£®
½ā“š ½ā£ŗÓÉĶ¼ĻóæÉÖŖ£¬“ÓæŖŹ¼ÖĮ¼ÓČėNaOHČÜŅŗ20mL£¬Ć»ÓŠ³ĮµķÉś³É£¬ĖµĆ÷ŌČÜŅŗÖŠĮņĖįČܽāMg”¢AlŗóĮņĖįÓŠŹ£Óą£¬“ĖŹ±·¢ÉśµÄ·“Ó¦ĪŖ£ŗH2SO4+2NaOH=Na2SO4+2H2O£®µ±V£ØNaOHČÜŅŗ£©=200mLŹ±£¬³ĮµķĮæ×ī“󣬓ĖŹ±ĪŖMg£ØOH£©2ŗĶAl£ØOH£©3£¬¶žÕßĪļÖŹµÄĮæÖ®ŗĶĪŖ0.35mol£¬ČÜŅŗÖŠČÜÖŹĪŖNa2SO4£¬øł¾ŻÄĘŌŖĖŲŹŲŗćæÉÖŖ“ĖŹ±n£ØNa2SO4£©µČÓŚ200mLĒāŃõ»ÆÄĘČÜŅŗÖŠŗ¬ÓŠµÄn£ØNaOH£©µÄ$\frac{1}{2}$±¶£®“Ó200mLµ½240mL£¬NaOHČܽāAl£ØOH£©3£ŗNaOH+Al£ØOH£©3=NaAlO2+2H2O£¬µ±V£ØNaOHČÜŅŗ£©=240mLŹ±£¬³Įµķ²»ŌŁ¼õÉŁ£¬“ĖŹ±Č«²æĪŖMg£ØOH£©2£¬ĪļÖŹµÄĮæĪŖ0.15mol£¬ĖłŅŌ³ĮµķĮæ×ī“ó£¬Mg£ØOH£©2ĪŖ0.15mol£¬Al£ØOH£©3ĪŖ0.35mol-0.15mol=0.2mol£¬ÓÉÓŚ“Ó200mLµ½240mL£¬NaOHČܽāAl£ØOH£©3£ŗNaOH+Al£ØOH£©3=NaAlO2+2H2O£¬ĖłŅŌøĆ½×¶ĪĻūŗÄn£ØNaOH£©=n[Al£ØOH£©3]=0.2mol£¬ĒāŃõ»ÆÄʵÄÅضČĪŖ$\frac{0.2mol}{0.24L-0.20L}$=5mol/L£¬
A£®ÓÉŌŖĖŲŹŲŗćæÉÖŖn£ØAl£©=n[Al£ØOH£©3]=0.2mol£¬n£ØMg£©=n[Mg£ØOH£©2]=0.15mol£¬ŌņĆ¾ŗĶĀĮµÄ×ÜÖŹĮæĪŖ0.2mol”Į27g/mol+0.15mol”Į24g/mol=9g£¬¹ŹAÕżČ·£»
B£®ÓÉĶ¼ĻóæÉÖŖ£¬“ÓæŖŹ¼ÖĮ¼ÓČėNaOHČÜŅŗ20mL£¬Ć»ÓŠ³ĮµķÉś³É£¬ĖµĆ÷ŌČÜŅŗÖŠĮņĖįČܽāMg”¢AlŗóĮņĖįÓŠŹ£Óą£¬“ĖŹ±·¢ÉśµÄ·“Ó¦ĪŖH2SO4+2NaOH=Na2SO4+2H2O£¬¹ŹBÕżČ·£»
C£®³ĮµķĮæ×ī“󣬓ĖŹ±ĪŖMg£ØOH£©2ŗĶAl£ØOH£©3£¬ČÜŅŗÖŠČÜÖŹĪŖNa2SO4£¬øł¾ŻÄĘŌŖĖŲŹŲŗćæÉÖŖ“ĖŹ±n£ØNa2SO4£©µČÓŚ200mLĒāŃõ»ÆÄĘČÜŅŗÖŠŗ¬ÓŠµÄn£ØNaOH£©µÄ$\frac{1}{2}$±¶£¬ĖłŅŌn£ØNa2SO4£©=$\frac{1}{2}$”Į0.2L”Į5mol/L=0.5mol£¬ĖłŅŌĮņĖįµÄÅضČĪŖ$\frac{0.5mol}{0.2L}$=2.5mol/L£¬¹ŹC“ķĪó£»
D£®ÓÉAÖŠæÉÖŖn£ØAl£©=0.2mol£¬n£ØMg£©=0.15mol£¬øł¾Żµē×Ó×ŖŅĘŹŲŗćæÉÖŖ2n£ØH2£©=3n£ØAl£©+2n£ØMg£©=3”Į0.2mol+2”Į0.15mol=0.9mol£¬ĖłŅŌn£ØH2£©=0.45mol£¬¹ŹĒāĘųĢå»żĪŖ0.45mol”Į22.4mol/L=10.08L£¬¹ŹD“ķĪó£»
¹ŹŃ”D£®
µćĘĄ ±¾ĢāŅŌĶ¼ĻóĢāµÄŠĪŹ½æ¼²éĆ¾ĀĮµÄÖŲŅŖ»ÆŗĻĪļµÄŠŌÖŹ¼°¼ĘĖć£¬ĪŖøßĘµæ¼µć£¬°ŃĪÕĶ¼ĻóÖŠø÷½×¶ĪµÄĪļÖŹµÄĮæµÄ¹ŲĻµ¼°ø÷½×¶Ī·¢ÉśµÄ»Æѧ·“Ó¦ĪŖ½ā“šµÄ¹Ų¼ü£¬×¢ŅāĄūÓĆŹŲŗć¼ĘĖć£¬²ąÖŲ·ÖĪöÓė¼ĘĖćÄÜĮ¦µÄ×ŪŗĻ漲飬ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ¼ŗ¶žĖį
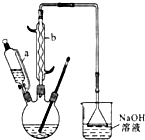
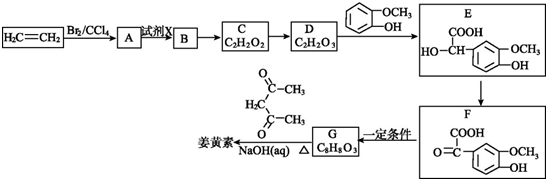
¼ŗ¶žĖį ŹĒŅ»ÖÖ¹¤ŅµÉĻ¾ßÓŠÖŲŅŖŅāŅåµÄÓŠ»ś¶žŌŖĖį£¬ŌŚ»Æ¹¤Éś²ś”¢ÓŠ»śŗĻ³É¹¤Ņµ”¢Ņ½Ņ©”¢Č󻬼ĮÖĘŌģµČ·½Ćę¶¼ÓŠÖŲŅŖ×÷ÓĆ£¬Äܹ»·¢Éś³ÉŃĪ·“Ó¦”¢õ„»Æ·“Ó¦µČ£¬²¢ÄÜÓė¶žŌŖ“¼Ėõ¾Ū³Éøß·Ö×Ó¾ŪŗĻĪļµČ£¬¼ŗ¶žĖį²śĮæ¾ÓĖłÓŠ¶žŌŖōČĖįÖŠµÄµŚ¶žĪ»£®ŹµŃéŹŅŗĻ³É¼ŗ¶žĖįµÄ·“Ó¦ŌĄķŗĶŹµŃé×°ÖĆŹ¾ŅāĶ¼ČēĻĀ£ŗ
ŹĒŅ»ÖÖ¹¤ŅµÉĻ¾ßÓŠÖŲŅŖŅāŅåµÄÓŠ»ś¶žŌŖĖį£¬ŌŚ»Æ¹¤Éś²ś”¢ÓŠ»śŗĻ³É¹¤Ņµ”¢Ņ½Ņ©”¢Č󻬼ĮÖĘŌģµČ·½Ćę¶¼ÓŠÖŲŅŖ×÷ÓĆ£¬Äܹ»·¢Éś³ÉŃĪ·“Ó¦”¢õ„»Æ·“Ó¦µČ£¬²¢ÄÜÓė¶žŌŖ“¼Ėõ¾Ū³Éøß·Ö×Ó¾ŪŗĻĪļµČ£¬¼ŗ¶žĖį²śĮæ¾ÓĖłÓŠ¶žŌŖōČĖįÖŠµÄµŚ¶žĪ»£®ŹµŃéŹŅŗĻ³É¼ŗ¶žĖįµÄ·“Ó¦ŌĄķŗĶŹµŃé×°ÖĆŹ¾ŅāĶ¼ČēĻĀ£ŗ
| ĪļÖŹ | ĆÜ¶Č£Ø20”ę£© | ČŪµć | ·Šµć | ČܽāŠŌ | Ļą¶Ō·Ö×ÓÖŹĮæ |
| »·¼ŗ“¼ | 0.962g/cm3 | 25.9”ę | 160.8”ę | 20”ꏱĖ®ÖŠČܽā¶Č3.6g£¬æÉ»ģČÜÓŚŅŅ“¼”¢±½ | 100 |
| ŅŅ¶žĖį | 1.36g/cm3 | 152”ę | 337.5”ę | ŌŚĖ®ÖŠµÄČܽā¶Č£ŗ15”ꏱ£¬1.44g£¬25”ꏱ2.3g£¬Ņ×ČÜÓŚŅŅ“¼£¬²»ČÜÓŚ±½ | 146 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® |  | B£® |  | C£® |  | D£® |  |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
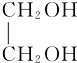 +O2$”ś_{”÷}^{Cu}$OHC-CHO+2H2O£®
+O2$”ś_{”÷}^{Cu}$OHC-CHO+2H2O£®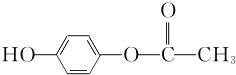 £®
£®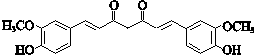 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ĄķĀŪÉĻČĪŗĪ×Ō·¢µÄŃõ»Æ»¹Ō·“Ó¦¶¼æÉŅŌÉč¼Ę³ÉŌµē³Ų£®ĻÖŹ¹ÓĆŠæµē¼«”¢Ķµē¼«”¢³Č×Ó£ØĖįŠŌ½éÖŹ£©Éč¼ĘČēĶ¼ĖłŹ¾µÄĖ®¹ūµē³Ų£®
ĄķĀŪÉĻČĪŗĪ×Ō·¢µÄŃõ»Æ»¹Ō·“Ó¦¶¼æÉŅŌÉč¼Ę³ÉŌµē³Ų£®ĻÖŹ¹ÓĆŠæµē¼«”¢Ķµē¼«”¢³Č×Ó£ØĖįŠŌ½éÖŹ£©Éč¼ĘČēĶ¼ĖłŹ¾µÄĖ®¹ūµē³Ų£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
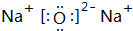 £®
£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com