
·ÖĪö £Ø1£©ĶʬµÄĻ”ŃĪĖįÖŠ¼ÓČėH2O2ŗó£¬ĶʬČܽā£¬·¢ÉśŃõ»Æ»¹Ō·“Ӧɜ³ÉĀČ»ÆĶ”¢Ė®£»
£Ø2£©¢ŁøßĆĢĖį¼ŲČÜŅŗ¾ßÓŠĒæŃõ»ÆŠŌ£¬Ėį»ÆµÄČÜŅŗ²»Äܱ»øßĆĢĖį¼ŲŃõ»Æ£»
¢ŚNa2S2O3ČÜŅŗĻŌ¼īŠŌ£»
¢ŪĄūÓĆ8MnO4-+5S2O32-+14H+=8Mn2++10SO42-+7H2O¼ĘĖć£®
½ā“š ½ā£ŗ£Ø1£©½žÅŻĶʬµÄĻ”ŃĪĖįÖŠ¼ÓČėH2O2ŗó£¬ĶʬČܽā£¬·¢ÉśŃõ»Æ»¹Ō·“Ӧɜ³ÉĀČ»ÆĶ”¢Ė®£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£¬Cu+2H++H2O=Cu2++2H2O£¬
¹Ź“š°øĪŖ£ŗCu+2H++H2O=Cu2++2H2O£»
£Ø2£©¢ŁøßĆĢĖį¼ŲČÜŅŗ¾ßÓŠĒæŃõ»ÆŠŌ£¬Ėį»ÆµÄČÜŅŗ²»Äܱ»øßĆĢĖį¼ŲŃõ»Æ£¬¶ŌKMnO4ČÜŅŗ½ųŠŠĖį»ÆŹ±£¬Ń”ÓƵÄĖįŹĒĮņĖį£¬¹Ź“š°øĪŖ£ŗĮņĖį£»
¢ŚNa2S2O3ČÜŅŗÖŠŅõĄė×ÓĖ®½āČÜŅŗĻŌ¼īŠŌ£¬ŌņÓ¦Ź¢·ÅŌŚ¼īŠŌµĪ¶Ø¹ÜÖŠ£¬¹Ź“š°øĪŖ£ŗ¼īŹ½£»
¢Ūn£ØNa2S2O3£©=0.012L”Į0.1mol/L=0.0012mol£¬Ōņ
8MnO4-+5S2O32-+14H+=8Mn2++10SO42-+7H2O
8 5
x 0.0012mol
x=0.00192mol£¬øßĆĢĖį¼ŲµÄÖŹĮæĪŖ0.00192mol”Į158g/mol=0.30336g£¬
ĖłŅŌĘäѳʷ֊KMnO4µÄ“æ¶ČŹĒ$\frac{0.30336g}{0.474g}$”Į100%=64%£¬
¹Ź“š°øĪŖ£ŗ64%£®
µćĘĄ ±¾Ģāæ¼²éÖŠŗĶµĪ¶ØŹµŃ锢Ńõ»Æ»¹Ō·“Ó¦·ÖĪöÅŠ¶Ļ£¬ĪŖøßĘµæ¼µć£¬°ŃĪÕ·¢ÉśµÄŃõ»Æ»¹Ō·“Ó¦”¢ŌŖĖŲµÄ»ÆŗĻ¼Ū±ä»Æ¼°µē×ÓŹŲŗćÓ¦ÓĆĪŖ½ā“šµÄ¹Ų¼ü£¬×¢ŅāĄė×ӵĊŌÖŹ¼°¼ĘĖćÓ¦ÓĆ£¬ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ijĪŽÉ«ČÜŅŗÖŠæÉÄÜ“óĮæ“ęŌŚH+”¢Cl-”¢MnO4- | |
| B£® | “óĮæĒāĄė×ÓµÄČÜŅŗÖŠæÉÄÜ“óĮæ“ęŌŚNa+”¢NH4+”¢SiO32- | |
| C£® | Fe2+ÓėH2O2ŌŚĖįŠŌČÜŅŗÖŠµÄ·“Ó¦£ŗ2Fe2++H2 O2+2H+ØT2Fe3++2H2O | |
| D£® | Ļ”ĮņĖįÓėBa£ØOH£©2ČÜŅŗµÄ·“Ó¦£ŗH++SO42-+Ba2++OH-ØTBaSO4”ż+2H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŗģÄ«Ė®¼Óµ½ĒåĖ®Ź¹Õū±Ė®±äŗģ | B£® | ±łŌŚŹŅĪĀĻĀČŚ»Æ³ÉĖ® | ||
| C£® | Ė®Ķłøß“¦Į÷ | D£® | ĢśĘ÷ŌŚ³±ŹŖµÄæÕĘųÖŠÉśŠā |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ·“Ó¦NH4HCO3£Øs£©ØTNH3£Øg£©+H2O£Øg£©+CO2£Øg£©”÷H=185.57 kJ•mol-1ÄÜ×Ō·¢½ųŠŠ£¬ŹĒŅņĪŖĢåĻµÓŠ×Ō·¢µŲĻņ»ģĀŅ¶ČŌö“óµÄ·½Ļņ×Ŗ±äµÄĒćĻņ | |
| B£® | ÄÜ×Ō·¢½ųŠŠµÄ·“Ó¦Ņ»¶ØÄÜŃøĖŁ·¢Éś | |
| C£® | ŅņĪŖģŹ±äŗĶģŲ±ä¶¼Óė·“Ó¦µÄ×Ō·¢ŠŌÓŠ¹Ų£¬Ņņ“ĖģŹ±ä»ņģŲ±ä¾łæÉŅŌµ„¶Ą×öĪŖÅŠ¶Ļ·“Ó¦ÄÜ·ń×Ō·¢½ųŠŠµÄÅŠ¾Ż | |
| D£® | CaCO3£Øs£©ØTCaO£Øs£©+CO2£Øg£©”÷H£¾0£¬”÷S£¾0£¬²»ĀŪŌŚŗĪÖÖĢõ¼žĻĀ¶¼æÉÄÜ×Ō·¢ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
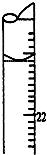 ѧɜÓĆ0.1000mol•L-1±ź×¼ĒāŃõ»ÆÄĘČÜŅŗµĪ¶ØĪ“ÖŖÅØ¶ČµÄŃĪĖįČÜŅŗ£¬Ęä²Ł×÷æÉ·ÖĪŖ£ŗ
ѧɜÓĆ0.1000mol•L-1±ź×¼ĒāŃõ»ÆÄĘČÜŅŗµĪ¶ØĪ“ÖŖÅØ¶ČµÄŃĪĖįČÜŅŗ£¬Ęä²Ł×÷æÉ·ÖĪŖ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
| Ę½ŠŠŹµŃ鱹ŗÅ | Na2C2O4ČÜŅŗ £ØmL£© | µĪ¶Ø¹ÜĘšŹ¼¶ĮŹż£ØmL£© | µĪ¶Ø¹ÜµĪ¶ØÖÕµć¶ĮŹż£ØmL£© |
| 1 | 20.00 | 0.00 | 21.18 |
| 2 | 20.00 | 1.02 | 21.00 |
| 3 | 20.00 | 1.18 | 21.20 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| Ćū³Ę | ĶŠÅĢĢģĘ½ | Š”ÉÕ± | ŪįŪöĒÆ | ²£Į§°ō | Ņ©³× | ĮæĶ² |
| ŅĒĘ÷ |  |  |  |  |  | |
| ŠņŗÅ | a | b | c | d | e | f |
| ŹµŃé “ĪŹż | ĘšŹ¼ĪĀ¶Čt1/”ę | ÖÕÖ¹ĪĀ¶Č t2/”ę | ĪĀ¶Č²īĘ½¾łÖµ £Øt2-t1£©/”ę | ||
| H2SO4 | NaOH | Ę½¾łÖµ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
| ŹµŃé“ĪŹż | µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī |
| “ż²āŹ³“×µÄĢå»ż³õ¶ĮŹż/mL | 0.02 | 0.03 | 0.00 |
| “ż²āŹ³“×µÄĢå»żÖÕ¶ĮŹż/mL | 25.01 | 25.04 | 25.02 |
| ĒāŃõ»ÆÄʱź×¼ŅŗµÄĢå»ż³õ¶ĮŹż/mL | 0.01 | 0.03 | 0.04 |
| ĒāŃõ»ÆÄʱź×¼ŅŗµÄĢå»żÖÕ¶ĮŹż/mL | 12.52 | 12.55 | 12.58 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com