
һ���¶��£���3molA�����1molB����ͨ��һ�ܱ������У��������·�Ӧ��
3A(g)+B (g) xC(g)������д���пհף�
xC(g)������д���пհף�
��1������������̶�Ϊ2L����Ӧ1minʱ���ʣ��1.8molA��C��Ũ��Ϊ0.4mol/L��
��1min�ڣ�B��ƽ����Ӧ����Ϊ____________________________��xֵΪ_____________��
������Ӧ��2min�ﵽƽ�⣬ƽ��ʱC��Ũ��_________0.8mol/L(����ڡ��������ڡ���С�ڡ�)��
��ƽ�������У�C���������Ϊ22%����A��ת������_____________��
�ܸı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ���������ԭƽ����ȣ���ʼ������������ʵ����ʵ���n(A)��n(B)��n(C)֮��Ӧ����Ĺ�ϵʽ___________________�� ��
��2����ά������ѹǿ����
�ٴﵽƽ��ʱC���������_________22%��(����ڡ��������ڡ���С�ڡ�)��
�ڸı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ�����ԭƽ���2������Ӧ����_________molA�����_________molB���塣
��1����0.2 mol/(L��min)�� 2 ���� ��36%
��n(A)+3n(C)/2=3 n(B)+ n(C)/2=1��
��2���� ���� �� 6�� 2
����������1��������A��1.2mol�����Ը��ݷ���ʽ��֪������B��0.4mol������1min�ڣ�B��ƽ����Ӧ����Ϊ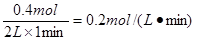 ��C��Ũ��Ϊ0.4mol/L��������C��0.8mol�����Ը��ݷ�Ӧ����֮������Ӧ�Ļ�ѧ������֮�ȿ�֪��x��2��
��C��Ũ��Ϊ0.4mol/L��������C��0.8mol�����Ը��ݷ�Ӧ����֮������Ӧ�Ļ�ѧ������֮�ȿ�֪��x��2��
���������ŷ�Ӧ�Ľ��У���Ӧ���Ũ����С�����Է�Ӧ�������ͣ��������Ӧ��2min�ﵽƽ�⣬ƽ��ʱC��Ũ��С��0.8mol/L��
��������A��3amol��������B��amol������C��2amol�������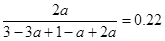 �����a��0.36������A��ת������3a��3��0.36����36����
�����a��0.36������A��ת������3a��3��0.36����36����
���������¶Ⱥ������ݻ����䣬����Ҫ����ƽ���Ч��������֮��A��B�����ʵ���Ӧ�÷ֱ����3mol��1mol������n(A)��n(B)��n(C)֮��Ӧ����Ĺ�ϵʽ��n(A)+3n(C)/2=3��n(B)+ n(C)/2=1��
��2������������Ӧ�������С�ģ������������ѹǿ���䣬��������ƽ��������Ӧ������У����Դﵽƽ��ʱC�������������22%��
���������¶Ⱥ�ѹǿ���䣬���������Чƽ�������������֮��A��B�����ʵ���֮�ȵ���3�U1�������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ�����ԭƽ���2������Ӧ����6molA�����2molB���塣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 3 |
| 2 |
| 1 |
| 2 |
| 3 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 3 |
| 2 |
| 1 |
| 2 |
| 3 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 3 |
| 2 |
| 1 |
| 2 |
| 3 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��������ѧ�߶���һ���¿���DZ�ࣩܰ��ѧ�Ծ����������� ���ͣ�������
һ���¶��£���3molA�����1molB����ͨ��һ�ܱ������У��������·�Ӧ��
3A(g)+B (g) xC(g)������д���пհף�
xC(g)������д���пհף�
��1������������̶�Ϊ2L����Ӧ1minʱ���ʣ��1.8molA��C��Ũ��Ϊ0.4mol/L��
��1min�ڣ�B��ƽ����Ӧ����Ϊ____________________________��xֵΪ_____________��
������Ӧ��2min�ﵽƽ�⣬ƽ��ʱC��Ũ��_________0.8mol/L(����ڡ��������ڡ���С�ڡ�)��
��ƽ�������У�C���������Ϊ22%����A��ת������_____________��
�ܸı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ���������ԭƽ����ȣ���ʼ������������ʵ����ʵ���n(A)��n(B)��n(C)֮��Ӧ����Ĺ�ϵʽ___________________�� ��
��2����ά������ѹǿ����
�ٴﵽƽ��ʱC���������_________22%��(����ڡ��������ڡ���С�ڡ�)��
�ڸı���ʼ���ʼ����������ʹ��Ӧ�ﵽƽ��ʱC�����ʵ�����ԭƽ���2������Ӧ����_________molA�����_________molB���塣
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com