
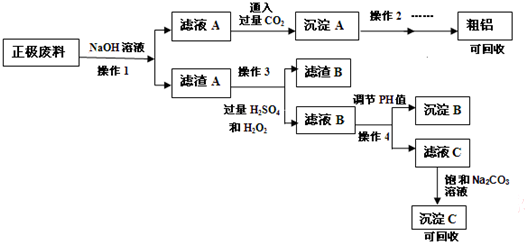
���� ����ӵ���������ϣ��ɷ�ΪLiFePO4��̼�ۺ�����������������Һ�ܽ⣬���˵�����A�к���LiFePO4��̼�ۣ���ҺA�к���ƫ�����ƣ���ҺA��ͨ�����������̼�ó���AΪ�������������������������շֽ⡢���ɵ���������A�м���˫��ˮ�����ᣬ���˵�����BΪ̼�ۣ���ҺB����Ҫ�ɷ�ΪFe3+��Li+��PO43-��������ҺB��PHֵ�����ˣ��ɵó���BΪ������������ҺC�к���Li+��PO43-����ҺC�м���̼���ƿɵó���CΪLi2CO3���ݴ˴��⣮
��� �⣺����ӵ���������ϣ��ɷ�ΪLiFePO4��̼�ۺ�����������������Һ�ܽ⣬���˵�����A�к���LiFePO4��̼�ۣ���ҺA�к���ƫ�����ƣ���ҺA��ͨ�����������̼�ó���AΪ�������������������������շֽ⡢���ɵ���������A�м���˫��ˮ�����ᣬ���˵�����BΪ̼�ۣ���ҺB����Ҫ�ɷ�ΪFe3+��Li+��PO43-��������ҺB��PHֵ�����ˣ��ɵó���BΪ������������ҺC�к���Li+��PO43-����ҺC�м���̼���ƿɵó���CΪLi2CO3��
��1�������Һ�����Ӧʹ�ù��ˣ��ʴ�Ϊ�����ˣ�
��2����ҺA�к���ƫ�����ƣ�ͨ�����������̼�õ�����AΪ����������������̼�����ƣ����ӳ�ʽΪ��CO2+2H2O+NaAlO2=NaHCO3+Al��OH��3����
�ʴ�Ϊ��CO2+2H2O+NaAlO2=NaHCO3+Al��OH��3����
��3������Ӱ�췴Ӧ���ʵ����ؿ�֪����߲���1�����ʵķ��������£����裬����Ũ�ȣ�����ʱ��ȣ�
�ʴ�Ϊ�����£����裬����Ũ�ȣ�����ʱ��ȣ�
��4������3�м���˫��ˮ�����������������������ӣ���Ӧ�����ӷ���ʽΪ��2LiFePO4+H2O2+2H+=2Fe3++2Li++2PO43-+2H2O��
�ʴ�Ϊ��2LiFePO4+H2O2+2H+=2Fe3++2Li++2PO43-+2H2O��
��5��﮵�س��ʱ��������Ϊ����������������Ӧ����ӦʽΪLiFePO4 -e-=FePO4+Li+��
�ʴ�Ϊ��LiFePO4 -e-=FePO4+Li+��
��6������4���ǵ���PHֵ��ʹ�������ӳ�����ȫ��Ӧѡ���Լ�ΪNaOH��
�ʴ�Ϊ��NaOH��
��7����������ķ�����֪������C�ijɷ�ΪLi2CO3��
�ʴ�Ϊ��Li2CO3��
���� ����Ϊ���������⣬�漰�����Ļ��ա�������ԭ��Ӧ�����ʵķ��롢������ұ�������⣬��Ŀ��Ϊ�ۺϣ�����ʱע����ϸ���⣬����Ŀ�л�ȡ�ؼ���Ϣ���Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
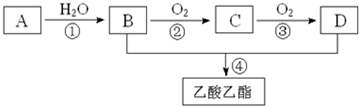
 ������������21�����������ȼ�ϵ�������Դ����ش���������
������������21�����������ȼ�ϵ�������Դ����ش����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3-CH=CH2��HCl�ӳ� | |
| B�� | CH3-CH2-CHOH-CH3��Ũ��������£�������������ˮ | |
| C�� | C6H5-CH3�����۴�������������Ӧ | |
| D�� | CH3-CHCl-CH3���������ƴ���Һ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ʰ���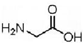 ���������� CH3CH2CH2NO2��Ϊͬ���칹�� ���������� CH3CH2CH2NO2��Ϊͬ���칹�� | |
| B�� | ������ͬͨʽ�������л��Ҫô��Ϊͬϵ��Ҫô��Ϊͬ���칹�� | |
| C�� | C3H9N������ | |
| D�� | �л�����̼ԭ����һ��ʱ����������ԭ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������з�������Ҫ��ӦΪCl2+2Br-�TBr2+2Cl- | |
| B�� | ����ڢ۵�Ŀ���Ǹ�����Ԫ�� | |
| C�� | ����X ΪHBrO | |
| D�� | �����������õ������ӷ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
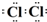 ���÷�Ӧ�����ӷ���ʽ��MnO2+4H++2Cl-$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++Cl2��+2H2O��
���÷�Ӧ�����ӷ���ʽ��MnO2+4H++2Cl-$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++Cl2��+2H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com