



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ˮ����ͭ�����Ҵ����Ƿ�ˮ |
| B������ˮ�����������Ƿ��в�����֬���� |
| C��������ķ������Է���������Ҵ��Ļ���� |
| D������ij±�������Ƿ��壺����NaOH��Һ�����ȣ���ȴ���ټ�����������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ���� | ���е����� | ���е����� |
| 1 | ���۵⻯����Һ | Ũ���� |
| 2 | ��̪��Һ | Ũ���� |
| 3 | �Ȼ�����Һ | Ũ��ˮ |
| 4 | ʪ��ĺ�ֽ | ������ˮ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ����������� | ʵ����� |
| A | ��ij��Һ�м��������ữ���Ȼ�����Һ���а�ɫ�������� | ��Һ��һ������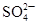 |
| B | ��ij��Һ�м������ᣬ������ʹ����ʯ��ˮ����ǵ���ɫ���� | ��Һ��һ������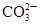 |
| C | ����ȼƲⶨSO2��CO2������Һ��pH��ǰ��pHС | H2SO3����ǿ��H2CO3 |
| D | �ò�����պȡŨ��ˮ�㵽��ɫʯ����ֽ�ϣ���ֽ����ɫ | Ũ��ˮ�ʼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��C2H6�л��е�C2H4�����Խ����������ͨ����ˮ��Ȼ���ü�ʯ�Ҹ��� |
| B��CaCO3�л��е�����NaHCO3�������ü��ȵķ�����ȥ |
| C����ϩ�л��е�SO2���壬����ͨ�����Ը��������Һ��ȥ |
| D��H2S�л��е�ˮ������������Ũ�����ȥ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ�鲽�� | ��������� |
| ����1 �����Թ��м��������������ټ�������_________________������� | ����Һ��ɫ���Ըı䣬����_______���ɣ���֤���������ʴ��� |
| ����2�� ������1�еõ�����Һ���ˣ���������ˮϴ����ϴ��Һ��ɫ | |
| ����3��ȡ����2�õ��������������Թ��У� �μ�___________________________________ _______________________________________ | __________________________________ ___________________________________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
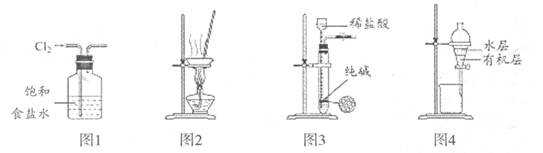
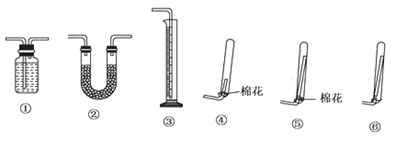
| A����ͼ1װ�ó�ȥC12��������HCl |
| B����ͼ2װ������NH4Cl������Һ��ȡNH4Cl���� |
| C����ͼ3װ����ȡ������CO2���� |
| D����ͼ4װ�÷���CCl4��ȡ��ˮ����л����ˮ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NO����������NO2����ˮϴ�Ӻ��ٸ��� |
| B��ʳ������������NH4Cl���ӹ������ռ���Һ��������� |
| C��N2����������O2��ͨ�����ȵ�ͭ�� |
| D��CO2�л���������SO2���壺ͨ������NaHCO3��Һϴ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com