
| ŹµŃ鱹ŗÅ | V£ØÉÕ¼īČÜŅŗ£©/mL | V£ØHCl£©/mL | |
| ³õ¶ĮŹż | Ä©¶ĮŹż | ||
| 1 | 20.00 | 0.60 | 20.62 |
| 2 | 20.00 | 0.80 | 20.78 |
| 3 | 20.00 | 0.20 | 20.90 |
·ÖĪö £Ø1£©øł¾ŻĖį¼īÖøŹ¾¼Į±äÉ«µÄpH·¶Ī§·ÖĪö£ŗ¢Ł¼×»ł³Č3.1”«4.4 ¢Ś¼×»łŗģ4.4”«6.2 ¢Ū·ÓĢŖ8.2”«10£¬Ń”ŌńŗĻŹŹµÄÖøŹ¾¼Į£¬×¢Ņā²»ÄÜČĆĢ¼Ėį±µÓėŃĪĖį·“Ó¦£»
øł¾ŻµĪ¶ØĒ°ČÜŅŗĪŖŗģÉ«£¬µĪ¶Ø½įŹųŹ±ČÜŅŗĪŖĪŽÉ«ÅŠ¶ĻµĪ¶ØÖÕµćĻÖĻó£»
£Ø2£©øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©•V£Ø±ź×¼£©}{V£Ø“ż²ā£©}$·ÖĪöµĪ¶Ø²Ł×÷¶Ō²ā¶Ø½į¹ūµÄÓ°Ļģ£»
£Ø3£©øł¾ŻČż“ĪµĪ¶ØĻūŗĵÄŃĪĖįĢå»ż£¬ĻČÅŠ¶ĻŹż¾ŻµÄÓŠŠ§ŠŌ£¬Č»ŗó¼ĘĖć³öĻūŗÄŃĪĖįµÄĘ½¾łĢå»ż£¬ŌŁøł¾Ż·“Ó¦¼ĘĖć³ö“ż²āŅŗĒāŃõ»ÆÄʵÄÅØ¶Č¼°ŃłĘ·ÖŠNaOHµÄÖŹĮæ·ÖŹż£»
½ā“š ½ā£ŗ£Ø1£©øł¾ŻĢāÖŠĢį¹©µÄĖį¼īÖøŹ¾¼ĮµÄ±äÉ«·¶Ī§æÉÖŖ£¬Ö»ÓŠ·ÓĢŖµÄ±äÉ«ŌŚ¼īŠŌ·¶Ī§ÄŚ£¬“ĖŹ±Ö»ÓŠNaOHÓėHCl·“Ó¦£¬BaCO3²»ÓėHCl·“Ó¦£»
ŌČÜŅŗĪŖ¼īŠŌ£¬·ÓĢŖĪŖŗģÉ«£¬µĪ¶ØÖÕµćŹ±£¬“ļµ½ÖÕµćµÄĻÖĻóĪŖČÜŅŗµÄŃÕÉ«øÕŗĆÓÉĒ³ŗģ±äĪŖĪŽÉ«ĒŅ°ė·ÖÖÓÄŚŃÕÉ«²»±ä»Æ£»
¹Ź“š°øĪŖ£ŗ·ÓĢŖ£»×īŗóŅ»µĪŃĪĖįµĪČėŗó£¬ČÜŅŗÓÉĒ³ŗģÉ«±äĪŖĪŽÉ«ĒŅ30Ćė²»øı䣻
£Ø2£©A£®×¶ŠĪĘæĪ“ÓĆ“ż²āŅŗČóĻ“£¬¶Ō“ż²āŅŗĪļÖŹµÄĮæƻӊӰĻģ£¬²»Ó°ĻģµĪ¶Ø½į¹ū£¬¹ŹA“ķĪó£»
B£®ĖįŹ½µĪ¶Ø¹ÜĪ“ÓƱź×¼ŅŗČóĻ“£¬µ¼ÖĀ±ź×¼ŅŗÅØ¶Č¼õŠ”£¬Ļūŗĵıź×¼ŅŗĢå»żĘ«“ó£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©•V£Ø±ź×¼£©}{V£Ø“ż²ā£©}$æÉÖŖ£¬²ā¶Ø½į¹ūĘ«øߣ¬¹ŹBÕżČ·£»
C£®ŌŚµĪ¶ØĒ°ÓŠĘųÅŻ£¬µĪ¶ØŗóĘųÅŻĻūŹ§£¬Ļūŗĵıź×¼ŅŗĢå»żĘ«“ó£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©•V£Ø±ź×¼£©}{V£Ø“ż²ā£©}$æÉÖŖ£¬²ā¶Ø½į¹ūĘ«øߣ¬¹ŹCÕżČ·£»
D£®µĪ¶ØĒ°Ę½ŹÓ¶ĮŹż£¬µĪ¶Ø½įŹųø©ŹÓ¶ĮŹż£¬µ¼ÖĀ¶Į³öµÄ±ź×¼ŅŗĢå»żĘ«Š”£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©•V£Ø±ź×¼£©}{V£Ø“ż²ā£©}$æÉÖŖ£¬²ā¶Ø½į¹ūĘ«µĶ£¬¹ŹD“ķĪó£»
E£®µĪ¶ØÖÕµćĒ°¼ÓĖ®ĒåĻ“׶ŠĪĘ棬¶Ō“ż²āŅŗĪļÖŹµÄĮæƻӊӰĻģ£¬²»Ó°ĻģµĪ¶Ø½į¹ū£¬¹ŹE“ķĪó£»
F£®ÖøŹ¾¼Į±äÉ«ŗóĮ¢¼“¶ĮŹż£¬µ¼ÖĀ¶Į³öµÄ±ź×¼ŅŗĢå»żĘ«Š”£¬øł¾Żc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©•V£Ø±ź×¼£©}{V£Ø“ż²ā£©}$æÉÖŖ£¬²ā¶Ø½į¹ūĘ«µĶ£¬¹ŹF“ķĪó£»
¹ŹŃ”BC£®
£Ø3£©ŹµŃé1”¢2”¢3µÄµ½“ļµĪ¶ØÖÕµćŹ±ĖłŗÄHClČÜŅŗµÄ·Ö±šĢå»żĪŖ£ŗ20.02mL£¬19.98mL£¬22.70mL£¬ĖłŅŌµŚČż“ĪµĪ¶ØĪŖĪŽŠ§Źż¾Ż£¬ĻūŗÄŃĪĖįµÄĘ½¾łĢå»żĪŖ£ŗ$\frac{20.02mL+19.98mL}{2}$=20.00mL£¬“ż²āŅŗĒāŃõ»ÆÄʵÄÅضČc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©•V£Ø±ź×¼£©}{V£Ø“ż²ā£©}$=$\frac{20.00mL”Į0.2000mol•{L}^{-1}}{20.00mL}$=0.2mol/L£¬
×¼Č·³ĘČ”2.0gѳʷÅäÖĘ³É200mLČÜŅŗ£¬Ōņ20.00mLČÜŅŗÖŠŗ¬ÓŠŃłĘ·0.2g£¬20.00mLČÜŅŗÖŠĒāŃõ»ÆÄʵÄÖŹĮæĪŖ0.2mol/L”Į0.02L”Į40g/mol=0.16g£¬ŃłĘ·ÖŠNaOHµÄÖŹĮæ·ÖŹż¦Ų£ØNaOH£©=$\frac{0.16g}{0.2g}$”Į100%=80%£»
¹Ź“š°øĪŖ£ŗ80%£»
µćĘĄ ±¾Ģāæ¼²éĮĖĖį¼īÖŠŗĶŹµŃéµÄÓŠ¹ŲÅŠ¶Ļ”¢Īó²ī·ÖĪö£¬øĆĢāŹĒÖŠµČÄѶȵďŌĢā£¬ŹŌĢā×ŪŗĻŠŌĒ棬עÖŲÄÜĮ¦µÄÅąŃų£®øĆĢāµÄÄѵćŌŚÓŚĪó²ī·ÖĪö£¬Īó²ī·ÖĪöµÄ×ÜŅĄ¾ŻĪŖc£Ø“ż²ā£©=$\frac{c£Ø±ź×¼£©•V£Ø±ź×¼£©}{V£Ø“ż²ā£©}$æÉÖŖ£¬c£Ø“ż²ā£©µÄ“óŠ”Č”¾öÓŚV£Ø±ź×¼£©µÄ“󊔣®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŅŅĖįŅŅõ„£ØŅŅĖį£©£ŗÓĆNaOHČÜŅŗĻ“µÓŗó·ÖŅŗ | |
| B£® | ŅŅĶé£ØŅŅĻ©£©£ŗÓĆäåĖ®Ļ“Ęų | |
| C£® | äå±½£Øä壩£ŗÓĆNaOHČÜŅŗĻ“µÓŗó·ÖŅŗ | |
| D£® | ŅŅ“¼£ØĖ®£©£ŗÓĆÉśŹÆ»ŅĪüĖ®ŗóÕōĮó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
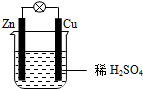
| A£® | CuĪŖøŗ¼«£¬ZnĪŖÕż¼« | B£® | µē×Ó“ÓĶʬ¾ĶāµēĀ·Į÷ĻņŠæʬ | ||
| C£® | øŗ¼«·“Ó¦ĪŖ£ŗZn-2e-ØTZn2+ | D£® | øĆ×°ÖĆ½«µēÄÜ×Ŗ»ÆĪŖ»ÆѧÄÜ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
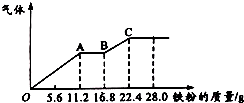 ijĻ”ĮņĖįŗĶĻ”ĻõĖįµÄ»ģŗĻČÜŅŗ200mL£¬Ę½¾ł·Ö³ÉĮ½·Ż£®ĻņĘäÖŠŅ»·ŻÖŠÖš½„¼ÓČėĶ·Ū£¬×ī¶ąÄÜČܽā19.2g£ØŅŃÖŖĻõĖįÖ»±»»¹ŌĪŖNOĘųĢ壩£®ĻņĮķŅ»·ŻÖŠÖš½„¼ÓČėĢś·Ū£¬²śÉśĘųĢåµÄĮæĖęĢś·ŪÖŹĮæŌö¼ÓµÄ±ä»ÆČēĶ¼ĖłŹ¾£®ĻĀĮŠ·ÖĪö»ņ½į¹ū“ķĪóµÄŹĒ£Ø””””£©
ijĻ”ĮņĖįŗĶĻ”ĻõĖįµÄ»ģŗĻČÜŅŗ200mL£¬Ę½¾ł·Ö³ÉĮ½·Ż£®ĻņĘäÖŠŅ»·ŻÖŠÖš½„¼ÓČėĶ·Ū£¬×ī¶ąÄÜČܽā19.2g£ØŅŃÖŖĻõĖįÖ»±»»¹ŌĪŖNOĘųĢ壩£®ĻņĮķŅ»·ŻÖŠÖš½„¼ÓČėĢś·Ū£¬²śÉśĘųĢåµÄĮæĖęĢś·ŪÖŹĮæŌö¼ÓµÄ±ä»ÆČēĶ¼ĖłŹ¾£®ĻĀĮŠ·ÖĪö»ņ½į¹ū“ķĪóµÄŹĒ£Ø””””£©| A£® | »ģŗĻĖįÖŠH2SO4ÅضČĪŖ4 mol•L-1 | |
| B£® | 200mL»ģŗĻĖįÖŠNO3-ĪļÖŹµÄĮæĪŖ0.2mol | |
| C£® | AB¶ĪµÄ·“Ó¦ĪŖFe+2Fe3+ØT3Fe2+£¬BC¶Ī²śÉśĒāĘų | |
| D£® | ¼ÓČėĶ·ŪµÄÄĒ·Ż»ģŗĻĖį£¬·“Ó¦ŗóŹ£Óą0.1mol H2SO4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 2ÖÖ | B£® | 3ÖÖ | C£® | 4ÖÖ | D£® | 6ÖÖ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
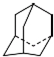 £¬ÓĆŅ»øöĀČŌ×ÓÓėŅ»øöäåŌ×ÓČ”“śŗóŠĪ³ÉµÄ¶žŌŖČ”“śĪļŹżÄæĪŖ£Ø””””£©
£¬ÓĆŅ»øöĀČŌ×ÓÓėŅ»øöäåŌ×ÓČ”“śŗóŠĪ³ÉµÄ¶žŌŖČ”“śĪļŹżÄæĪŖ£Ø””””£©| A£® | 8 | B£® | 7 | C£® | 6 | D£® | 5 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĶÖ¬Ė®½āæÉÉś³Éøß¼¶Ö¬·¾ĖįŗĶ±ūČż“¼ | |
| B£® | µķ·ŪĖ®½āµÄ×īÖÕ²śĪļÄÜÓėŠĀÖʵÄCu£ØOH£©2Šü×ĒŅŗ·“Ó¦ | |
| C£® | µķ·ŪŗĶĻĖĪ¬ĖŲµÄ·Ö×ÓŹ½¾łæÉÓĆ£ØC6H10O5£©n±ķŹ¾£¬ĖūĆĒ»„ĪŖĶ¬·ÖŅģ¹¹Ģå | |
| D£® | µ°°×ÖŹČÜŅŗÖŠ¼ÓČė±„ŗĶĮņĖįļ§ČÜŅŗŗó²śÉśµÄ³ĮµķÄÜÖŲŠĀČÜÓŚĖ® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ąė×Ó»ÆŗĻĪļÖŠŅ»¶ØÖ»ŗ¬ÓŠĄė×Ó¼ü | |
| B£® | µ„ÖŹ·Ö×ÓÖŠ¾ł“ęŌŚ»Æѧ¼ü | |
| C£® | ½öŗ¬ÓŠ¹²¼Ū¼üµÄ»ÆŗĻĪļŅ»¶ØŹĒ¹²¼Ū»ÆŗĻĪļ | |
| D£® | Óɲ»Ķ¬ÖÖ·Ē½šŹōŌŖĖŲµÄŌ×ÓŠĪ³ÉµÄ¹²¼Ū»ÆŗĻĪļŅ»¶ØÖ»ŗ¬¼«ŠŌ¼ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
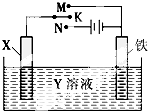 ĄūÓĆČēĶ¼×°ÖĆ£¬ÄÜĶź³Éŗܶąµē»ÆѧŹµŃ飮ĻĀĮŠÓŠ¹Ų“Ė×°ÖƵĊšŹöÖŠ£¬“ķĪóµÄŹĒ
ĄūÓĆČēĶ¼×°ÖĆ£¬ÄÜĶź³Éŗܶąµē»ÆѧŹµŃ飮ĻĀĮŠÓŠ¹Ų“Ė×°ÖƵĊšŹöÖŠ£¬“ķĪóµÄŹĒ| A£® | ČōXĪŖŠæ°ō£¬YĪŖNaClČÜŅŗ£¬æŖ¹ŲKÖĆÓŚM“¦£¬æɼõ»ŗĢśµÄøÆŹ“£¬ÕāÖÖ·½·Ø³ĘĪŖĪžÉüŃō¼«µÄŅõ¼«±£»¤·Ø | |
| B£® | ČōXĪŖĢ¼°ō£¬YĪŖNaClČÜŅŗ£¬æŖ¹ŲKÖĆÓŚN“¦£¬æɼõ»ŗĢśµÄøÆŹ“£¬ÕāÖÖ·½·Ø³ĘĪŖĶā¼ÓµēĮ÷µÄŅõ¼«±£»¤·Ø | |
| C£® | ČōXĪŖĶ°ō£¬YĪŖĮņĖįĶČÜŅŗ£¬æŖ¹ŲKÖĆÓŚM“¦£¬Ģśµē¼«ÉĻµÄµē¼«·“Ó¦Ź½ĪŖCu2++2e-ØTCu | |
| D£® | ČōXĪŖĶ°ō£¬YĪŖĮņĖįĶČÜŅŗ£¬æŖ¹ŲKÖĆÓŚN“¦£¬ČÜŅŗÖŠø÷Ąė×ÓÅØ¶Č¶¼²»»į±ä»Æ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com