��������1�����ø�˹���������㷴Ӧ�ȣ����û�ѧƽ����������ȡ������������ж���ѧƽ�⣬�������η�����ƽ��ʱ��Ũ�������㻯ѧƽ�ⳣ����
��2������Ũ���̺�ƽ�ⳣ����С�Ƚ��жϷ�Ӧ���еķ�����Q=K����Ӧ�ﵽƽ�⣬Q��K����Ӧ������У�Q��K��Ӧ������У�ʹ�ø÷�Ӧ�Ļ�ѧ��Ӧ���ʼӿ죬ͬʱʹƽ��ʱNH
3������ٷ������ӣ���Ҫ�ı����� ���㻯ѧ��Ӧ����������ƽ��������У�
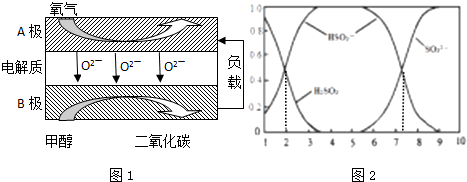
��3��B������״�������������Ӧ��Ϊ��صĸ������ܷ�ӦʽΪ2CH
3OH+3O
2=2CO
2+4H
2O��������ӦʽΪ��O
2+2e
-=2O
2-����ʽ����ɵø����缫��Ӧʽ��
��ʼ�η�����Ӧ��2Cu
2++2H
2O
2Cu+O
2��+4H
+��ͭ������ȫ�ŵ������Ӧ2H
2O
2H
2��+O
2�����������ռ���������������ʱ���������������������ȣ���������Ϊxmol�����ݵ���ת���غ��з��̼��㣬�ٸ��ݵ���ת���غ�������ĵļ״�������
��4������ͼ2��֪��
a��pH=8ʱ����Һ��c��HSO
3-����c��SO
32-������a��ȷ��
b��pH=2ʱ��c��HSO
3-��=c��H
2SO
3�����ٽ�ϵ���غ㣺c��Na
+��+c��H
+��=c��HSO
3-��+2c��SO
32-��+c��OH
-�����ɵ���Һ��c��Na
+��+c��H
+��=c��H
2SO
3��+2c��SO
32-��+c��OH
-����
c����Һ�� c��SO
32-��=c��HSO
3-�� ʱ��PH��7����c��H
+����c��OH
-����
d��pH������4��5���ң���Ԫ�ؼ���ȫ����NaHSO
3��ʽ���ڣ�
���
�⣺��1�����ݸ�˹���ɣ����ڶ�������ʽ�ߵ����������һ������ʽ��ӵã�2NO
2+2SO
2�T2SO
3+2NO����H=-83.6 kJ?mol
-1����NO
2+SO
2?SO
3+NO����H=-41.8 kJ?mol
-1��
NO
2��g��+SO
2��g��?SO
3��g��+NO��g��
��ʼ���ʵ���� a 2a 0 0
ת�����ʵ���� x x x x
ƽ�����ʵ���� a-x 2a-x x x
��a-x������2a-x��=1��6����x=
a��
��ƽ�ⳣ��Ϊ=
=
��
�ʴ�Ϊ��-41.8��2.67��
��
��2��һ��ʱ���N
2��H
2��NH
3�����ʵ����ֱ�Ϊ2mol/L��1mol/L��2mol/Lʱ��Qc=
=0.5�����Ը�״̬��ƽ��״̬�����淴Ӧ������ȣ��ʴ�Ϊ��=��
��ʹ�ø÷�Ӧ�Ļ�ѧ��Ӧ���ʼӿ죬ͬʱʹƽ��ʱNH
3������ٷ������ӣ�
A����Ӧ�����������С�ķ�Ӧ����С�������ѹǿ����Ӧ��������ƽ��������У���A��ȷ��
B����Ӧ�Ƿ��ȷ�Ӧ�������¶ȣ���������ƽ��������У���B����
C���Ӵ����ı䷴Ӧ���ʣ����ı�ƽ�⣬��C����
D��ʹ����Һ�����ߣ�ƽ��������У���Ӧ���ʼ�С����D����
E�����º��������£���Ͷ��N
2��H
2��NH
3�����ʵ���2mol��1mol��2mol���൱�ڼ�ѹ��ʹ�ø÷�Ӧ�Ļ�ѧ��Ӧ���ʼӿ죬ͬʱʹƽ��ʱNH
3������ٷ������ӣ��ʴ�Ϊ��AE��
��3��B������״�������������Ӧ��Ϊ��صĸ������ܷ�ӦʽΪ2CH
3OH+3O
2=2CO
2+4H
2O��������ӦʽΪ��O
2+2e
-=2O
2-����ʽ�����������ӦΪ��CH
3OH+3O
2--6e
-=CO
2+2H
2O��
�ʴ�Ϊ��CH
3OH+3O
2--6e
-=CO
2+2H
2O��
��2������ͭ�����ʵ���=0.1L��1mol/L=0.1mol����ʼ�η�����Ӧ��2Cu
2++2H
2O
2Cu+O
2��+4H
+��ͭ������ȫ�ŵ������Ӧ2H
2O
2H
2��+O
2�����������ռ���������������ʱ���������������������ȣ���������Ϊxmol�����ݵ���ת���غ㣬��0.1mol��2+2x=4x�����x=0.1��
���ݵ���ת���غ㣬��֪���ĵļ״����ʵ���=
=0.067mol���ʴ�Ϊ��0.067mol����15�֣�
��4������ͼ2��֪��
a��pH=8ʱ����Һ��c��HSO
3-����c��SO
32-������a��ȷ��
b��pH=2ʱ��c��HSO
3-��=c��H
2SO
3�����ٽ�ϵ���غ㣺c��Na
+��+c��H
+��=c��HSO
3-��+2c��SO
32-��+c��OH
-�����ɵ���Һ��c��Na
+��+c��H
+��=c��H
2SO
3��+2c��SO
32-��+c��OH
-������b��ȷ��
c����Һ�� c��SO
32-��=c��HSO
3-�� ʱ��PH��7����c��H
+����c��OH
-������c����
d��Ϊ��þ����ܴ���NaHSO
3���ɽ���Һ��pH������4��5���ң���d��ȷ����ѡabd��




 ������λ�ڶ����ڵ��������ڡ���������ķǽ���Ԫ��X��Y����֪��Ԫ������������ˮ�����Ϊǿ�ᣮ������ͼת����ϵ����Ӧ���������ֲ�������ȥ�����ش��������⣺
������λ�ڶ����ڵ��������ڡ���������ķǽ���Ԫ��X��Y����֪��Ԫ������������ˮ�����Ϊǿ�ᣮ������ͼת����ϵ����Ӧ���������ֲ�������ȥ�����ش��������⣺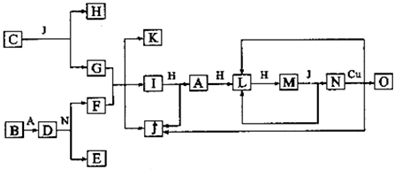
 ������Ϊ3��3��4-��������
������Ϊ3��3��4-��������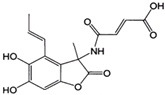 ��NaOH��Һ��Br2��Ӧʱ���ֱ���Ҫ����NaOH 5.0mol��Br2 3.0mol
��NaOH��Һ��Br2��Ӧʱ���ֱ���Ҫ����NaOH 5.0mol��Br2 3.0mol