
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ˮ��Ӧʮ��ƽ��������H2 |
| B��������ᷴӦ������CsSO4��H2 |
| C��蘆��������ֱ����ˮ��Ӧ����CsOH |
| D����ڿ�����ȼ������Cs2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��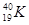 �� ��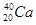 | B�����ʯ��ʯī | C��H2O��D2O | D��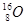 �� ��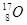 �� ��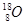 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
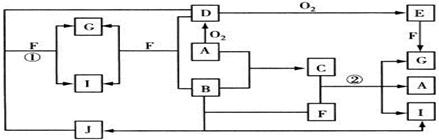
 ת����ϵ��ʡ����ˮ�Ͳ��ַ�Ӧ�P�����������Ӧ���⣬������Ӧ������Һ�н��С�D�ǹ�ҵ����õĽ�����
ת����ϵ��ʡ����ˮ�Ͳ��ַ�Ӧ�P�����������Ӧ���⣬������Ӧ������Һ�н��С�D�ǹ�ҵ����õĽ�����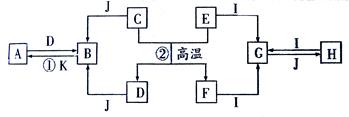
 ��
��| T(��) | 200 | 600 | 800 |
| ��(g/L) | 6��881 | 2��650 | 1��517 |
| ����Ħ�������L/mol�� | 38��8 | 71��6 | 88��0 |
 00��ʱ��ˮH ������ʽ�ķ���ʽΪ ��
00��ʱ��ˮH ������ʽ�ķ���ʽΪ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
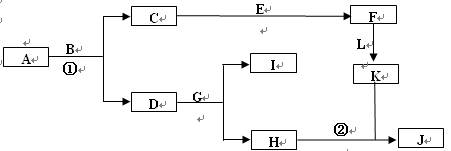
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
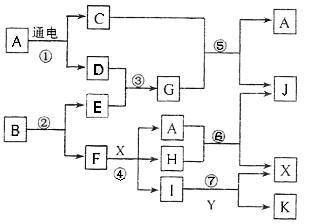
 �밴Ҫ����գ���1��д��A�ĵ���ʽ ��FԪ�������ڱ�λ�� ��2����Ӧ�ݵĻ�ѧ����ʽΪ�� ��
�밴Ҫ����գ���1��д��A�ĵ���ʽ ��FԪ�������ڱ�λ�� ��2����Ӧ�ݵĻ�ѧ����ʽΪ�� ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com