
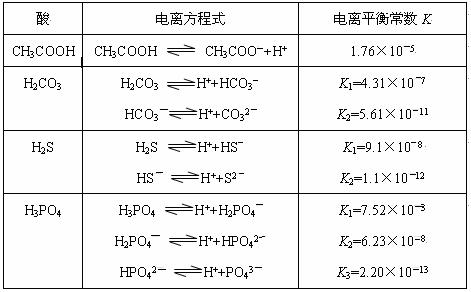
¶ФУЪИхЛб,ФЪТ»¶ЁОВ¶ИПВґпµЅµзАлЖЅєвК±,ёчОўБЈµДЕЁ¶ИґжФЪТ»ЦЦ¶ЁБїµД№ШПµЎЈИф25ЎжК±УРHA![]() H++AЁDЈ¬ФтK=
H++AЁDЈ¬ФтK=![]() ЎЈКЅЦРЈєKОЄµзАлЖЅєвіЈКэЈ¬Ц»УлОВ¶ИУР№ШЈ¬cОЄёчОўБЈµДЖЅєвЕЁ¶ИЎЈПВ±нКЗјёЦЦіЈјыИхЛбµДµзАлЖЅєвіЈКэЈЁ25ЎжЈ©ЎЈ
ЎЈКЅЦРЈєKОЄµзАлЖЅєвіЈКэЈ¬Ц»УлОВ¶ИУР№ШЈ¬cОЄёчОўБЈµДЖЅєвЕЁ¶ИЎЈПВ±нКЗјёЦЦіЈјыИхЛбµДµзАлЖЅєвіЈКэЈЁ25ЎжЈ©ЎЈ
»ШґрПВБРёчМвЈє
ЈЁ1Ј©KЦ»УлОВ¶ИУР№ШЈ¬µ±ОВ¶ИЙэёЯК±Ј¬KЦµ__________ЈЁМоЎ°ФцґуЎ±ЎўЎ°јхРЎЎ±ЎўЎ°І»±дЎ±Ј©ЎЈ
ЈЁ2Ј©ФЪОВ¶ИПаН¬К±Ј¬ёчИхЛбµДKЦµІ»Н¬Ј¬ДЗГґKЦµµДґуРЎУлЛбРФµДПа¶ФЗїИхУРєО№Ш Пµ?_____________________________ЎЈ
ЈЁ3Ј©Иф°СCH3COOHЎўH2CO3ЎўHCO3ЈЎўH2SЎўHSЈЎўH3PO4ЎўH2PO4ЈЎўHPO42Ј¶јїґЧчКЗЛбЈ¬ЖдЦРЛбРФЧоЗїµДКЗ_______Ј¬ЧоИхµДКЗ_____________ЎЈ
ЈЁ4Ј©¶аФЄИхЛбКЗ·ЦІЅµзАлµДЈ¬ГїТ»ІЅ¶јУРПаУ¦µДµзАлЖЅєвіЈКэЈ¬¶ФУЪН¬Т»ЦЦ¶аФЄИхЛбµДK1ЎўK2ЎўK3Ц®јдґжФЪЧЕКэБїЙПµД№жВЙЈ¬ґЛ№жВЙКЗ________________Ј¬ІъЙъґЛ№жВЙµДФТтКЗ___________________________________ЎЈ

| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
| C(A-)Ј®C(H+) |
| C(HA) |
| C(A-)Ј®C(H+) |
| C(HA) |
| Лб | µзАл·ЅіМКЅ | µзАлЖЅєвіЈКэK |
| CH3COOH | CH3COOH?CH3COOH-+H+ | 1.76ЎБ10-5 |
| H2CO3 | H2CO3?H++HCO3- HCO3-?H++HCO32- |
K1=4.31ЎБ10-7 K2=5.61ЎБ10-11 |
| H2S | H2S?H++HS- HS-?H++S2- |
K1=9.1ЎБ10-8 K2=1.1ЎБ10-12 |
| H3PO4 | H3PO4?H++H2PO4- H2PO4-H++HPO42- HPO42-?H++PO43- |
K1=7.52ЎБ10-3 K2=6.23ЎБ10-8 K3=2.20ЎБ10-13 |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈєФД¶БАнЅв
| Лб | µзАл·ЅіМКЅ | µзАлЖЅєвіЈКэK |
| CH3COOH | CH3COOH?CH3COO-+H+ | 1.76ЎБ10-5 |
| HClO | HClO?ClO-+H+ | 2.95ЎБ10-8 |
| H2S | H2S?H++HS- HS-?H++S2- |
K1=9.1ЎБ10-8 K2=1.1ЎБ10-12 |
| H2CO3 | H2CO3?H++HCO3- HCO3-?H++CO32- |
K1=4.31ЎБ10-7 K2=5.61ЎБ10-11 |
| H3PO4 | H3PO4?H++H2PO4- H2PO4-?H++HPO42- HPO42-?H++PO43- |
K1=7.1ЎБ10-3 K2=6.3ЎБ10-8 K3=4.2ЎБ10-13 |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
¶ФУЪИхЛбЈ¬ФЪТ»¶ЁОВ¶ИПВґпµЅµзАлЖЅєвК±Ј¬ёчОўБЈµДЕЁ¶ИґжФЪТ»ЦЦ¶ЁБїµД№ШПµЈ®ПВ±нКЗ25ЎжК±јёЦЦіЈјыИхЛбµДµзАлЖЅєвіЈКэ
| Лб | µзАл·ЅіМКЅ | µзАлЖЅєвіЈКэK |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
¶ФУЪИхЛбЈ¬ФЪТ»¶ЁОВ¶ИПВґпµЅµзАлЖЅєвК±Ј¬ёчОўБЈµДЕЁ¶ИґжФЪТ»ЦЦ¶ЁБїµД№ШПµЈ®ПВ±нКЗ25ЎжК±јёЦЦіЈјыИхЛбµДµзАлЖЅєвіЈКэ
| Лб | µзАл·ЅіМКЅ | µзАлЖЅєвіЈКэK |
| | | |
| | | |
| | | |
| | | |
»ШґрПВБРёчОКЈє
ЈЁ1Ј©KЦ»УлОВ¶ИУР№ШЈ¬µ±ОВ¶ИЙэёЯК±Ј¬KЦµ________ЈЁМоЎ°ФцґуЎ±ЎўЎ°јхРЎЎ±ЎўЎ°І»±дЎ±Ј©Ј®
ЈЁ2Ј©ФЪОВ¶ИПаН¬К±Ј¬ёчИхЛбµДKЦµІ»Н¬Ј¬ДЗГґKЦµµДґуРЎУлЛбРФµДПа¶ФЗїИхУРєО№ШПµ?__________________Ј®
ЈЁ3Ј©Иф°СCH3COOHЎўH2CO3ЎўHCO3-ЎўH2SЎўHS-ЎўH3PO4ЎўH2PO4-ЎўHPO42-¶јїґЧчКЗЛбЈ¬ЖдЦРЛбРФЧоЗїµДКЗ_________Ј¬ЧоИхµДКЗ________Ј®
ЈЁ4Ј©¶аФЄИхЛбКЗ·ЦІЅµзАлµДЈ¬ГїТ»ІЅ¶јУРПаУ¦µДµзАлЖЅєвіЈКэЈ®¶ФУЪН¬Т»ЦЦ¶аФЄИхЛбµДK1ЎўK2ЎўK3Ц®јдґжФЪЧЕКэБїЙПµД№жВЙЈ¬¶ФУЪH3PO4ґЛ№жВЙКЗ________________Ј¬ІъЙъґЛ№жВЙµДФТтКЗ_________________________Ј®
![]() ЈЁ5Ј©µзАлЖЅєвіЈКэКЗУГКµСйµД·Ѕ·ЁІв¶ЁіцАґµДЈ®ПЦТСѕІвµГДіОВ¶ИПВ NH3∙H2OИЬТєЦРґжФЪИзПВ·ґУ¦ЈєNH3∙H2O NH4++OH- ТСЦЄ0.10 molЎ¤L-1NH3∙H2OИЬТєЦРЈ¬ґпµЅЖЅєвК±Ј¬CЖЅєвЈЁOH-Ј©=4.2 ЎБ 10-3molЎ¤L-1Ј¬CЖЅєвЈЁNH3∙H2OЈ©ЎЦCЖрКјЈЁNH3∙H2OЈ©Ј¬Л®µДµзАлїЙєцВФІ»јЖ;
ЈЁ5Ј©µзАлЖЅєвіЈКэКЗУГКµСйµД·Ѕ·ЁІв¶ЁіцАґµДЈ®ПЦТСѕІвµГДіОВ¶ИПВ NH3∙H2OИЬТєЦРґжФЪИзПВ·ґУ¦ЈєNH3∙H2O NH4++OH- ТСЦЄ0.10 molЎ¤L-1NH3∙H2OИЬТєЦРЈ¬ґпµЅЖЅєвК±Ј¬CЖЅєвЈЁOH-Ј©=4.2 ЎБ 10-3molЎ¤L-1Ј¬CЖЅєвЈЁNH3∙H2OЈ©ЎЦCЖрКјЈЁNH3∙H2OЈ©Ј¬Л®µДµзАлїЙєцВФІ»јЖ;
ўЩУГpHКФЦЅІвБїИЬТєµДpHЦµЈ¬јґїЙЗуµГCЖЅєвЈЁOH-Ј©Ј¬Ів¶ЁИЬТєpHЦµµДІЩЧчКЗ______________ЎЈ
ўЪІвБїCЖЅєвЈЁNH3∙H2OЈ©µД·Ѕ·ЁЧоєГУГ_____________·ЁЈЁМо·Ѕ·ЁГыіЖЈ©
ўЫЗуґЛОВ¶ИПВёГ·ґУ¦µДЖЅєвіЈКэK.(РґіцјЖЛг№эіМЈ¬јЖЛгЅб№ы±ЈБф2О»УРР§КэЧЦ)
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє2010Дк№г¶«КЎёЯ¶юЙПС§ЖЪЖЪЦРїјКФ»ЇС§ѕн МвРНЈєМоїХМв
¶ФУЪИхЛбЈ¬ФЪТ»¶ЁОВ¶ИПВґпµЅµзАлЖЅєвК±Ј¬ёчОўБЈµДЕЁ¶ИґжФЪТ»ЦЦ¶ЁБїµД№ШПµЈ®ПВ±нКЗ25ЎжК±јёЦЦіЈјыИхЛбµДµзАлЖЅєвіЈКэ
|
Лб |
µзАл·ЅіМКЅ |
µзАлЖЅєвіЈКэK |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
»ШґрПВБРёчОКЈє
ЈЁ1Ј©KЦ»УлОВ¶ИУР№ШЈ¬µ±ОВ¶ИЙэёЯК±Ј¬KЦµ________ЈЁМоЎ°ФцґуЎ±ЎўЎ°јхРЎЎ±ЎўЎ°І»±дЎ±Ј©Ј®
ЈЁ2Ј©ФЪОВ¶ИПаН¬К±Ј¬ёчИхЛбµДKЦµІ»Н¬Ј¬ДЗГґKЦµµДґуРЎУлЛбРФµДПа¶ФЗїИхУРєО№ШПµ?__________________Ј®
ЈЁ3Ј©Иф°СCH3COOHЎўH2CO3ЎўHCO3-ЎўH2SЎўHS-ЎўH3PO4ЎўH2PO4-ЎўHPO42-¶јїґЧчКЗЛбЈ¬ЖдЦРЛбРФЧоЗїµДКЗ_________Ј¬ЧоИхµДКЗ________Ј®
ЈЁ4Ј©¶аФЄИхЛбКЗ·ЦІЅµзАлµДЈ¬ГїТ»ІЅ¶јУРПаУ¦µДµзАлЖЅєвіЈКэЈ®¶ФУЪН¬Т»ЦЦ¶аФЄИхЛбµДK1ЎўK2ЎўK3Ц®јдґжФЪЧЕКэБїЙПµД№жВЙЈ¬¶ФУЪH3PO4ґЛ№жВЙКЗ________________Ј¬ІъЙъґЛ№жВЙµДФТтКЗ_________________________Ј®
 ЈЁ5Ј©µзАлЖЅєвіЈКэКЗУГКµСйµД·Ѕ·ЁІв¶ЁіцАґµДЈ®ПЦТСѕІвµГДіОВ¶ИПВ NH3∙H2OИЬТєЦРґжФЪИзПВ·ґУ¦ЈєNH3∙H2O NH4++OH-
ТСЦЄ0.10 molЎ¤L-1
NH3∙H2OИЬТєЦРЈ¬ґпµЅЖЅєвК±Ј¬CЖЅєвЈЁOH-Ј©=4.2 ЎБ 10-3molЎ¤L-1Ј¬CЖЅєвЈЁNH3∙H2OЈ©ЎЦCЖрКјЈЁNH3∙H2OЈ©Ј¬Л®µДµзАлїЙєцВФІ»јЖ;
ЈЁ5Ј©µзАлЖЅєвіЈКэКЗУГКµСйµД·Ѕ·ЁІв¶ЁіцАґµДЈ®ПЦТСѕІвµГДіОВ¶ИПВ NH3∙H2OИЬТєЦРґжФЪИзПВ·ґУ¦ЈєNH3∙H2O NH4++OH-
ТСЦЄ0.10 molЎ¤L-1
NH3∙H2OИЬТєЦРЈ¬ґпµЅЖЅєвК±Ј¬CЖЅєвЈЁOH-Ј©=4.2 ЎБ 10-3molЎ¤L-1Ј¬CЖЅєвЈЁNH3∙H2OЈ©ЎЦCЖрКјЈЁNH3∙H2OЈ©Ј¬Л®µДµзАлїЙєцВФІ»јЖ;
ўЩУГpHКФЦЅІвБїИЬТєµДpHЦµЈ¬јґїЙЗуµГCЖЅєвЈЁOH-Ј©Ј¬Ів¶ЁИЬТєpHЦµµДІЩЧчКЗ______________ЎЈ
ўЪІвБїCЖЅєвЈЁNH3∙H2OЈ©µД·Ѕ·ЁЧоєГУГ_____________·ЁЈЁМо·Ѕ·ЁГыіЖЈ©
ўЫЗуґЛОВ¶ИПВёГ·ґУ¦µДЖЅєвіЈКэK.(РґіцјЖЛг№эіМЈ¬јЖЛгЅб№ы±ЈБф2О»УРР§КэЧЦ)
Ійїґґр°ёєНЅвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com