
(17��)Na2SO3�����ڿ����о������ױ��� ��
(I)Na2SO3�����ڿ����б��ʵ���Ҫԭ���û�ѧ����ʽ��ʾΪ ��
(II)Ϊ̽��Na2SO3��Ʒ�ı����������������¼��裺
����1��Na2SO3��Ʒ��ȫ���ʣ� ����2��Na2SO3��Ʒ��ȫû�б��ʣ�����3�� ��
�����������ʵ����̼�����ͽ��ۣ����̽����
| ʵ����� | ����ͽ��� |
| ����1��ȡ������Ʒ���Թ��У�������������ˮ����ܽ⣬�ٵμ�H2SO4�ữ��KMnO4��Һ�� | ������KMnO4��Һ���Ϻ�ɫ��Ϊ��ɫ �ٽ��ۣ���Ʒ���� ���ӣ�����1�������� ����ɫ�����ӷ���ʽΪ�� �� |
| ����2����ȡ������Ʒ���Թ��У�������������ˮ����ܽ⣬�ٵμ�ϡHCl��ʹ��Һ�����ԣ��ٵμ�����BaCl2��Һ�� | �������� �� ���ۣ�����2������ |
| ���� | ���� |
(I) 2Na2SO3+ O2 ==2Na2SO4��
(II)����3�� Na2SO3���ֱ��� ����SO32-���ӣ� ��5SO32-+2MnO4-+ 6H+ =5SO42- +2Mn2++3H2O��
��������������������壬��BaCl2�ް�ɫ�������� ��
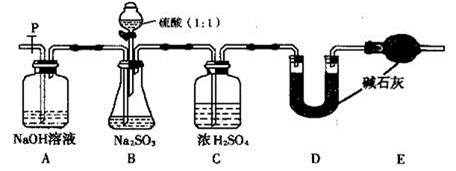
(III)��1�� �رտ���P�ͷ�Һ©����������C�м�ˮ����û���ܿڣ���B��C���ܿ�������ð����ֹͣ����һ��������γɵ�����ˮ���������������á�
��2�� ��װ���е�SO2ȫ������U�ι������ա� ��3�� D ��
�������������(I)��Na2SO3��S�Ļ��ϼ�Ϊ+4�ۣ����ױ������е���������Ϊ+6�۵�Na2SO4.��Ӧ�ķ���ʽ��2Na2SO3+ O2 ==2Na2SO4��(II)������Ŀ�Ѿ�������֪ʶ��֪������3��Na2SO3���ֱ��ʣ���H2SO4�ữ��KMnO4��Һ��ǿ�����ԣ����뻹ԭ�Ե����ӷ�����Ӧ����ɫ�����ۣ���Ʒ����SO32-���ӣ�֤������1���������ڷ�����ɫ�����ӷ���ʽΪ��5SO32-+2MnO4-+ 6H+ =5SO42- +2Mn2++3H2O����ȡ������Ʒ���Թ��У�������������ˮ����ܽ⣬�ٵμ�ϡHCl��ʹ��Һ�����ԣ��ٵμ�����BaCl2��Һ������������������壬��BaCl2�ް�ɫ�������ɣ���֤������SO32-��SO42-����.����2������(III)��1�����Bװ�������ԵIJ���Ϊ�رտ���P�ͷ�Һ©����������C�м�ˮ����û���ܿڣ���B��C���ܿ�������ð����ֹͣ����һ��������γɵ�����ˮ���������������á���2��ʵ���д���ƿ�в��ٲ����������P�ӵ�����˻�������һ�����Ŀ�������������Ŀ���ǽ�װ���е�SO2ȫ������U�ι������գ��Լ�Сʵ������3�����ѳ�����a g Na2SO3��Ʒ�⣬ʵ���л�Ӧ�ⶨ��������D��U�ι���������ǰ���������ֵ��
���㣺����װ�õ������Եļ�顢���ӵļ��顢���ӷ���ʽ����д��ʵ�鷽������ơ��о������۵�֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
Ũ����������У������ԡ���ǿ�����ԡ�����ˮ�ԡ����ѻӷ��Եȡ��Ҵ����廯�ƺ�Ũ�����Ϲ����Ʊ�������ʱ�����ж������Ӧ�������˹�����Ũ������ʾ��������
| A���٢ڢۢ� | B���ٺ͢� | C��ֻ�Т� | D���ٺ͢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��12�֣���ͼ��ʾ������֮���ת����ϵ����֪��������B��D��F��G��I��J�����壬 F��G�ǿ����е���Ҫ�ɷ֣�D��һ�ּ������塣A�����������Ӹ�������1��1�� E��һ�ֺ�ɫ�����H���Ϻ�ɫ�������ʡ�(����������ͷ�Ӧ����ʡ��)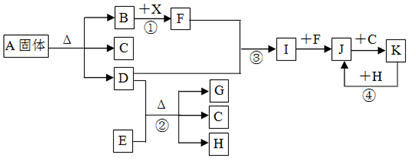
�밴Ҫ����գ�
��1��A���ʵĻ�ѧʽ�� ��
��2����Ӧ�۵Ļ�ѧ����ʽ�� ��
��Ӧ�ܵ����ӷ���ʽ�� ��
��3����Ӧ���в�����״����1.12L����F����ת�Ƶĵ�����Ŀ�� ��
��4��������ҺA�������ӵķ�����
��
��5����Ӧ������������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
1��92gCuƬ��һ������ŨHNO3���ã����ռ���NO2��NO���干1��12Lʱ����״����������ͭǡ��ȫ�����á���
��1����Ӧ������HNO3 mol��ת�Ƶ��� mol
��2������ˮ���ռ����ɵ�����,�����������Ϊ L (��״��)
��3�����ռ�����������ͨ�� mL O2(��״��)����ʹˮ�պó�����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����12�֣�
������Ȼ����������̬�Ͷ��ֻ���̬�γɳ��֡���Ļ���������������Ի�ԭ�ԡ������������������ˮ��
���������գ�
(1)������л�ԭ�ԣ����Ժ�������������Ӧ�������������£�H2S��KMnO4��Ӧ����S��MnSO4��K2SO4��H2O��д���÷�Ӧ�Ļ�ѧ����ʽ��_________________________________
(2)ʯ�ͻ����ķ�������H2S��д���ӷ����л��յ���������ַ������������⣬��ʹ������ԭ�ϣ����Ի�ѧ����ʽ��ʾ��_____________________��______________________
(3)�����£�0.1mol/L��������Һ��0.1mol.L��̼������Һ�����Ը�ǿ����_______����ԭ����________��
��֪��H2S��Ki1��1.3��10��7 Ki2��7.1��10��15
H2CO3��Ki1��4.3��10��7 Ki2��5.6��10��11
(4)��ZnSO4��Һ�еμӱ���H2S��Һ��û�г������ɣ������μ�һ�����İ�ˮ������ZnS�������õ���ƽ��ԭ��������������__________________________
(5)����ɫ��Fe2S3����������������У���Һ���е���ɫ�������ɣ����ﻹ��____��______�����ˣ�����Һ��Ȼ��������������������Һ���ɹ۲쵽��������______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��11�� ijУ��ѧ��ȤС���ͬѧ�������ε����ȷֽ����̽�����������������װ�÷ֱ������Ca��NO3��2��Cu(NO3)2��AgNO3���ֹ��塣�����ȼ��г�װ��δ������
��1����ͬѧ���ȵ���Ca��NO3��2�����ȹ��̷��֣�װ�â��в��� ���ݣ�����ʯ����Һ��ѹ��װ�â��У��ô����ǵ�ľ��������е����壬ľ����ȼ������װ�â���ʣ��Ĺ����֪��ʣ������к���NԪ�أ����ԣ�3�ۡ���д��Ca��NO3��2���ȷֽ�����ɲ���Ļ�ѧʽ�� �� ��
��2����ͬѧ���ȵ���Cu(NO3)2�����ȹ��̷��֣�װ�â���Ҳ�����ݲ��������������Ĺ�������ʧ��ʯ����Һ��Ϊ��ɫ��Һ�弸������ѹ��װ�â��С�װ�â��еĹ�����Ϊ��ɫ����д��Cu(NO3)2���ȷֽ�Ļ�ѧ����ʽ�� ��
��3����ͬѧ���ȵ���AgNO3�����ȹ��̷��֣�װ�â���Ҳ�����ݲ��������������Ĺ��������ݲ�����ʧ��ʣ�������Ҳ��ʹ�����ǵ�ľ����ȼ��ʯ����ҺҲ��Ϊ��ɫ��������Һ�屻ѹ��װ�â��С�װ�â��еĹ�����Ϊ��ɫ����ͬѧ�ݴ�д����AgNO3���ȷֽ���ܵ����ֻ�ѧ����ʽ��
����4AgNO3  2Ag2O��4NO2����O2�� ����2AgNO3
2Ag2O��4NO2����O2�� ����2AgNO3 2Ag��2NO2����O2����
2Ag��2NO2����O2����
������ȷ���� ����˵�����ɣ� ��
�������һ����ʵ��֤����Ľ�������ȷ�ģ� ��
��4��������3��ʵ��Ľ���������Ʋ����������ȷֽ�Ĺ��ɣ� ��
��5����������ͬѧ����������ag��������������ȫ�ֽ��ȡ��Ͳ���Ϊbml�����������ķֽ��ʣ�____________�����������������ϵ���ɲ��û���С����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�о�С��ģ�ҵ������ƺ����ˮ���ⶨ������Ч�ʣ�������ͼ��ʾװ�ý���ʵ�顣��CN����Ũ��Ϊ0.2 mol��L��1�ĺ����ˮ100 mL��100 mL NaClO��Һ������������װ�â�������ƿ�У���ַ�Ӧ����Һ©������������100 mLϡH2SO4���رջ�����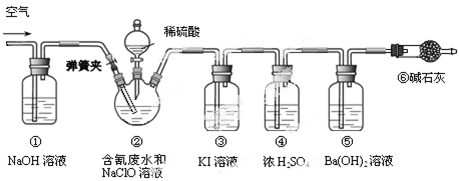
��֪װ�â��з�������Ҫ��Ӧ����Ϊ��
CN��+ ClO��=CNO��+ Cl�� 2CNO��+2H+ +3C1O��=N2��+2CO2��+3C1��+H2O
��1���ٺ͢������� ��
��2��װ�â��У���������װ�â۳�ȥ�����ʵ����ӷ���ʽΪ ��
��3����Ӧ��������ͨ�������Ŀ���� ��
��4��Ϊ�����ʵ���к����ˮ�������İٷ��ʣ���Ҫ�ⶨ ��������
��5����֪CN-�Ĵ���Ч�ʿɸߴ�90%��������CO2�ڱ�״���µ����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼÿһ�����е���ĸ����һ�ַ�Ӧ������������J�Ǻ�����Ԫ��A�İ�ɫ��״������IΪNaCl��Һ��D�ǵ���ɫ���嵥�ʡ�����д���пհס�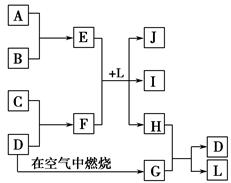
(1)��ͼ���������������ڷǵ���ʵ����ʵĻ�ѧʽ�� ��
(2)�õ���ʽ��ʾ��H���γɹ��� ��
(3)��E��ˮ��Һ���ɲ����յõ��Ĺ������ʵĻ�ѧʽΪ ��
(4)F��ˮ��Һ�и�����Ũ���ɴ�С��˳��Ϊ ��
(5)F��ˮ��Һ�Լ��Ե�ԭ�� (�����ӷ���ʽ��ʾ)��
(6)E��F��L�з�Ӧ�����ӷ���ʽΪ ��
(7)H��G֮�䷴Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
һͬѧ������Ԫ�صĻ��ϼ��У�2��0����4����6�������ʴ����м��̬��������Ӧ�ü��������ԣ����л�ԭ�ԡ���ͬѧ��̽����Ļ�ԭ�ԣ������Ǹ�ͬѧ��ʵ��̽�����̣��������е����⡣
(1)��ͬѧӦ��ѡ��________(�����������ԭ����)����ʵ��̽����
(2)��ͬѧ�ú��ȵIJ�������ȼ��ʯ�����ϵ���ۣ���ۿ�ʼȼ�գ���Ӧ�Ļ�ѧ����ʽΪ________________________________________________
(3)��ͬѧ����֤��ȼ�ղ�������ʣ����������ѡ����ʵ��Լ�����˵��ԭ��
| A�����Ƶ���ˮ | B��Ʒ����Һ | C�����з�̪��NaOHϡ��Һ | D��H2S���� |
| ���� | �Լ� | ��ѧ����ʽ |
| Ư���� | | |
| ������ | | |
| ��ԭ�� | | |
| ���������� | | |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com