
(6��)��һ��ϡ��Һ�������������֣���Һ��ɫ�����壬���п��ܺ���SO42����Na+��CO32�� ��H+��Cl���������е������֡�Ȼ������������ʵ�飬��ȷ����Щ�����Ƿ�������ڡ�
��1����pH��ֽ����Һ��pHֵ����ֽ�Ժ�ɫ��
��2��ȡ 2mL������Һ�������������ᱵ��Һ����������˲�����ϡ����İ�ɫ������
2mL������Һ�������������ᱵ��Һ����������˲�����ϡ����İ�ɫ������
��3��ȡ��2���е��ϲ���Һ��������������Һ������������˲�����ϡ����İ�ɫ������
�ʣ�����ʵ�����ȷ����Һ��һ������ ���ӣ�һ��û�� ���ӡ�
���ʵ�鷽�����鲻��ȷ�����������ӡ�
_____ ____________________________________________________________________
_________________________________________________________________________
 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��15�֣���Դ��ȱ���������ٵ��ش����⡣�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ������˼״�����Ϊ21���͵�����ȼ�ϡ�
��1����֪�ڳ��³�ѹ�£�
��2CH3OH(I)ʮ3O2(g) 2CO2(g)+4H2O(g) ��H= _1275.6 kJ��mol��1
��H2O(I) === H2O(g) ��H=+ 44.0 kJ.mol-1
д����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽ (2��).
��2����ҵ��һ������������ַ�Ӧ�ϳɼ״���
��ӦA��CO2��g��+3H2��g��CH3OH��g��+H2O��g�� ��H1
��ӦB��CO��g��+2H2��g��CH3OH��g�� ��H2
ȡ��ݵ����CO2��H2�Ļ�����壨���ʵ���֮�Ⱦ�Ϊ1��3�����ֱ�����¶Ȳ�ͬ���ݻ���ͬ�ĺ����ܱ������У�����������Ӧ����Ӧ��ͬʱ���ü״�����������գ�CH3OH���뷴Ӧ�¶�T�Ĺ�ϵ��������ͼ��ʾ��������CO2ת��Ϊ�״��ķ�Ӧ�ġ�H1________0�������������������������2�֣�
�ڶ��ڷ�ӦA���������ݻ����䣬���д�ʩ�����Ӽ״����ʵ��� ��2�֣���
A�������¶� B������CO2����
C������He��ʹ��ϵ��ѹǿ���� D����ԭ�����ٳ���CO2��H2
��ij�¶��£���4mol CO��12mol H2,����2L���ܱ������У���ַ�Ӧ���ﵽƽ����c(CO) =0.5 mol��L��1��������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ ��2�֣���
��.ij�ּ״�������ȼ�ϵ���Dz��ò���Ϊ�缫��ϡ�������������Һ���乤��ʱ�����ĵ缫��Ӧʽ�ɱ�ʾΪ______________________ ��2�֣�
��3����ȼú�����е�SO2��NO2�����ʵ���֮��Ϊ1��1,��һ�����İ�����������Ӧ����������狀�����淋Ļ������Ϊ����Ʒ���ʡ�����÷�Ӧ�Ļ�ѧ����ʽΪ________ ����3�֣���4����һ�����ʵ���Ũ�ȵ��������Һ�еμ�������NaOH��Һ��ʹ��Һ��pH=7����
��Һ��c��Na+��+c��H+��_____ c��NO3-��+c��OH-������д��������������������2�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(6��)��һ��ϡ��Һ�������������֣���Һ��ɫ�����壬���п��ܺ���SO42����Na+ ��CO32�� ��H+ ��Cl�� �������е������֡�Ȼ������������ʵ�飬��ȷ����Щ�����Ƿ�������ڡ�
��1����pH��ֽ����Һ��pHֵ����ֽ�Ժ�ɫ��
��2��ȡ2mL������Һ�������������ᱵ��Һ����������˲�����ϡ����İ�ɫ������
��3��ȡ��2���е��ϲ���Һ��������������Һ������������˲�����ϡ����İ�ɫ������
�ʣ�����ʵ�����ȷ����Һ��һ������ ���ӣ�һ��û�� ���ӡ�
���ʵ�鷽�����鲻��ȷ�����������ӡ�
_________________________________________________________________________
_________________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ����������ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
��15�֣���Դ��ȱ���������ٵ��ش����⡣�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ������˼״�����Ϊ21���͵�����ȼ�ϡ�
��1����֪�ڳ��³�ѹ�£�
��2CH3OH(I)ʮ3O2(g)
2CO2(g)+4H2O(g) ��H= _1275.6 kJ��mol��1
��H2O(I) === H2O(g) ��H=+ 44.0 kJ.mol-1
д����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽ (2��).
��2����ҵ��һ������������ַ�Ӧ�ϳɼ״���
��ӦA��CO2��g��+3H2��g�� CH3OH��g��+H2O��g�� ��H1
CH3OH��g��+H2O��g�� ��H1
��ӦB��CO��g��+2H2��g�� CH3OH��g�� ��H2
CH3OH��g�� ��H2
ȡ��ݵ����CO2��H2�Ļ�����壨���ʵ���֮�Ⱦ�Ϊ1��3�����ֱ�����¶Ȳ�ͬ���ݻ���ͬ�ĺ����ܱ������У�����������Ӧ����Ӧ��ͬʱ���ü״�����������գ�CH3OH�� �뷴Ӧ�¶�T�Ĺ�ϵ��������ͼ��ʾ��������CO2ת��Ϊ�״��ķ�Ӧ�ġ�H1________0�������������������������2�֣�
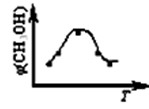
�ڶ��ڷ�ӦA���������ݻ����䣬���д�ʩ�����Ӽ״����ʵ��� ��2�֣���
A�������¶� B������CO2����
C������He��ʹ��ϵ��ѹǿ���� D����ԭ�����ٳ���CO2��H2
��ij�¶��£���4mol CO��12mol H2,����2L���ܱ������У���ַ�Ӧ���ﵽƽ����c(CO) =0.5 mol��L��1��������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ ��2�֣���
��.ij�ּ״�������ȼ�ϵ���Dz��ò���Ϊ�缫��ϡ�������������Һ���乤��ʱ�����ĵ缫��Ӧʽ�ɱ�ʾΪ______________________ ��2�֣�
��3����ȼú�����е�SO2��NO2�����ʵ���֮��Ϊ1��1,��һ�����İ�����������Ӧ����������狀�����淋Ļ������Ϊ����Ʒ���ʡ�����÷�Ӧ�Ļ�ѧ����ʽΪ________ ����3�֣���4����һ�����ʵ���Ũ�ȵ��������Һ�еμ�������NaOH��Һ��ʹ��Һ��pH=7����
��Һ��c��Na+��+c��H+��_____ c��NO3-��+c��OH-������д��������������������2�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���㽭ʡ������ֱ��УЭ�����һ��һѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(6��)��һ��ϡ��Һ�������������֣���Һ��ɫ�����壬���п��ܺ���SO42����Na+ ��CO32�� ��H+ ��Cl�� �������е������֡�Ȼ������������ʵ�飬��ȷ����Щ�����Ƿ�������ڡ�
��1����pH��ֽ����Һ��pHֵ����ֽ�Ժ�ɫ��
��2��ȡ2mL������Һ�������������ᱵ��Һ����������˲�����ϡ����İ�ɫ������
��3��ȡ��2���е��ϲ���Һ��������������Һ������������˲�����ϡ����İ�ɫ������
�ʣ�����ʵ�����ȷ����Һ��һ������ ���ӣ�һ��û�� ���ӡ�
���ʵ�鷽�����鲻��ȷ�����������ӡ�
_____ ____________________________________________________________________
_________________________________________________________________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com