
 ����ŨH2SO4�����ʵ���Ũ�ȣ�������Һϡ��ǰ�����ʵ��������������Ũ����������
����ŨH2SO4�����ʵ���Ũ�ȣ�������Һϡ��ǰ�����ʵ��������������Ũ���������� ���������������ľ��ȣ�ʵ�黷����
���������������ľ��ȣ�ʵ�黷����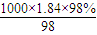 mol/L=18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL��
mol/L=18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL�� ���Լ������⣮
���Լ������⣮ 

 ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������500mL 0.2 mol/L��ϡ���
��1����Ҫ��ȡ��������Ϊ98%�����ᣨ�ܶ�Ϊ1.84 g��mL-1��_____________mL��
��2��������ijѧ���IJ������̣�
a.�������ƿ�Ƿ�©ˮ��b.��100 mL����Ͳ��ȡŨ���
c.��Ũ���ᵹ����һ��ʢ����������ˮ����Ͳ��ϡ�ͣ�����ȴ�����£�
d.�ò�������������ϡ�ͺ�����ᵹ��500 mL������ƿ��
e.����ҡ������ƿ��ʹƿ��Һ���Ͼ��ȣ���������ƿ�м�ˮ����̶���1 cm��2 cm;
f.�ý�ͷ�ιܼ�ˮ����Һ��ײ���̶������У�ҡ�ȣ�
g.������ƿ�����ϱ�ǩ���á�
����ͨ��������Ҫ��ָ������ȱ�ٻ�����Ĵ��������������м��������ո����Բ��ӣ���
��________________________________________________________��
��________________________________________________________��
��________________________________________________________��
��________________________________________________________��
��________________________________________________________��
��3���κ�ʵ�鶼�������������IJ����У���������ԭ����_________________����4����ʵ�����˷�ʱ��IJ����ǽ�ϡ�ͺ��������ȴ�����£�Ϊ�˽�Լʱ�䣬������еļӿ�ϡ������ȴ�ķ�����_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com