
| 1 |
| 2 |
| 1 |
| 2 |
| 3 |
| 2 |
| ��� | ԭ�����и���ֵ�������� | |||
| CO | CO2 | H2 | N2 | |
| ��1�� | 19.7 | 0.0 | 59.1 | 21.2 |
| ��2�� | 20.7 | 0.3 | 62.1 | 16.9 |
| ��3�� | 16.9 | 1.3 | 50.7 | 31.1 |
| ��4�� | 19.8 | 5.5 | 59.4 | 15.3 |
| ��5�� | 20.3 | 10.9 | 60.9 | 7.9 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2009?�Ͼ���ģ����ҵ�Ͽ����úϳ�����CO��H2�Ļ�����壩�����״����練Ӧ�ܣ�����֪��
��2009?�Ͼ���ģ����ҵ�Ͽ����úϳ�����CO��H2�Ļ�����壩�����״����練Ӧ�ܣ�����֪��| 1 |
| 2 |
| 1 |
| 2 |
| 3 |
| 2 |
| ��� | ԭ�����и���ֵ�������� | |||
| CO | CO2 | H2 | N2 | |
| ��1�� | 19.7 | 0.0 | 59.1 | 21.2 |
| ��2�� | 20.7 | 0.3 | 62.1 | 16.9 |
| ��3�� | 16.9 | 1.3 | 50.7 | 31.1 |
| ��4�� | 19.8 | 5.5 | 59.4 | 15.3 |
| ��5�� | 20.3 | 10.9 | 60.9 | 7.9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| 1 |
| 2 |
| 3 |
| 2 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���÷�Ӧ�ġ�S��0 | B���÷�Ӧ���κ��¶��¾����Է����� | C���������г�������He��ƽ��������Ӧ�����ƶ� | D�������¶�ƽ��������Ӧ�����ƶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��㶫ʡ��ɽ��������⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
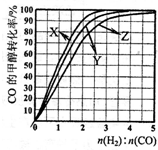
��ҵ�Ͽ����úϳ�����CO��H2�Ļ�����壩�����״�����֪��
CO(g)+2H2(g) CH3OH(g)
��H=��92.9kJ/mo1
CH3OH(g)
��H=��92.9kJ/mo1
һ�������£��÷�Ӧ��һ����̶����ܱ������дﵽƽ�⡣����˵����ȷ����
A���÷�Ӧ�ġ�S<0
B���÷�Ӧ���κ��¶��¾����Է�����
C���������г�������He��ƽ��������Ӧ�����ƶ�
D�������¶�ƽ��������Ӧ�����ƶ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com