
| A£® | 5.8gÓÉÕż¶”ĶéÓėŅģ¶”Ķé×é³ÉµÄ»ģŗĻĘųĢåÖŠŗ¬ÓŠµÄC-H¼üŹżÄæĪŖNA | |
| B£® | ½«0.2mol NH3³äČėČŻĘ÷ÖŠ¼ÓČČ·Ö½ā£¬Éś³ÉN2µÄ·Ö×ÓŹżĪŖ0.1NA | |
| C£® | 1L 0.1mol•L-1 NaHCO3ČÜŅŗÖŠHCO3-ŗĶCO32-ŹżÄæÖ®ŗĶĪŖ0.1NA | |
| D£® | µ±¹żĮæµÄĶÓėÅØHNO3·“Ӧɜ³É22.4LĘųĢåŹ±£¬×ŖŅʵĵē×ÓŹżĪŖNA |
·ÖĪö A”¢Ēó³ö»ģŗĻĪļµÄĪļÖŹµÄĮæ£¬Č»ŗóøł¾ŻÕż¶”ĶéŗĶŅģ¶”ĶéÖŠŗ¬ÓŠµÄC-H¼ü¾łĪŖ10ĢõĄ“·ÖĪö£»
B”¢°±ĘųµÄ·Ö½ā·“Ó¦ĪŖæÉÄę·“Ó¦£»
C”¢HCO3-¼ČÄܵēĄėĪŖCO32-ÓÖÄÜĖ®½āĪŖH2CO3£»
D”¢Éś³ÉĘųĢåĖł“¦µÄדĢ¬²»Ć÷Č·£®
½ā“š ½ā£ŗA”¢5.8gÕż¶”ĶéŗĶŅģ¶”ĶéµÄ»ģŗĻĪļµÄĪļÖŹµÄĮæĪŖ0.1mol£¬¶ųÕż¶”ĶéŗĶŅģ¶”ĶéÖŠŗ¬ÓŠµÄC-H¼ü¾łĪŖ10Ģõ£¬¹Ź0.1mol»ģŗĻĪļÖŠŗ¬ÓŠµÄC-H¼üĪŖNAĢõ£¬¹ŹAÕżČ·£»
B”¢°±ĘųµÄ·Ö½ā·“Ó¦ĪŖæÉÄę·“Ó¦£¬²»ÄܽųŠŠ³¹µ×£¬¹ŹÉś³ÉµÄµŖĘų·Ö×ÓøöŹżŠ”ÓŚ0.1NAøö£¬¹ŹB“ķĪó£»
C”¢HCO3-¼ČÄܵēĄėĪŖCO32-ÓÖÄÜĖ®½āĪŖH2CO3£¬øł¾ŻĪļĮĻŹŲŗćæÉÖŖ£¬ČÜŅŗÖŠHCO3-”¢CO32-”¢H2CO3Ö®ŗĶĪŖ0.1NAøö£¬¹ŹC“ķĪó£»
D”¢Éś³ÉĘųĢåĖł“¦µÄדĢ¬²»Ć÷Č·£¬¹ŹĘųĢåµÄĪļÖŹµÄĮæĪŽ·Ø¼ĘĖć£¬Ōņ×ŖŅʵĵē×ÓŹżĪŽ·Ø¼ĘĖć£¬¹ŹD“ķĪó£®
¹ŹŃ”A£®
µćĘĄ ±¾Ģāæ¼²éĮĖ°¢·üŁ¤µĀĀŽ³£ŹżµÄÓŠ¹Ų¼ĘĖć£¬ÄŃ¶Č²»“ó£¬Ó¦×¢ŅāÕĘĪÕ¹«Ź½µÄŌĖÓĆŗĶĪļÖŹµÄ½į¹¹£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 88 g | B£® | 44g/mol | C£® | 2 mol | D£® | ĪŽ·ØČ·¶Ø |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 1£ŗ2 | B£® | 1£ŗ4 | C£® | 2£ŗ1 | D£® | 4£ŗ1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Na ¼ČŹĒŃõ»Æ¼ĮÓÖŹĒ»¹Ō¼Į | B£® | NaHŹĒ»¹Ō²śĪļ | ||
| C£® | NaOH¼Č±»Ńõ»ÆÓÖ±»»¹Ō | D£® | Ćæ×ŖŅĘ1molµē×Ó£¬ŌņÉś³É1mol NaH |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NO | B£® | SO2 | C£® | O2 | D£® | CO |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŅņĪŖNaHSO4ŹĒµē½āÖŹ£¬Ņņ“Ė¹ĢĢåNaHSO4Äܹ»µ¼µē | |
| B£® | NaHSO4¹ĢĢåÖŠŃōĄė×ÓŗĶŅõĄė×ÓµÄøöŹż±ČŹĒ2£ŗ1 | |
| C£® | NaHSO4¹ĢĢåČŪ»ÆŹ±ĘĘ»µµÄŹĒĄė×Ó¼üŗĶ¹²¼Ū¼ü | |
| D£® | NaHSO4¹ĢĢåČÜÓŚĖ®Ź±¼ČĘĘ»µĄė×Ó¼üÓÖĘĘ»µ¹²¼Ū¼ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 2.4gĆ¾ŌŚ×ćĮæµÄO2”¢CO2”¢N2»ģŗĻĘųĢåÖŠČ¼ÉÕ£¬×ŖŅʵĵē×ÓŹżĪŖ0.1NA | |
| B£® | ±źæöĻĀ£¬11.2 LĀČĘųĶźČ«ČÜÓŚ1 LĖ®ÖŠ£¬ĖłµĆČÜŅŗÖŠCl-ŗĶClO-Į½ÖÖĮ£×ÓŹżÖ®ŗĶĪŖNA | |
| C£® | 0.1L0.5mol/LCH3COOHČÜŅŗÖŠŗ¬ÓŠµÄĒāĄė×ÓŹżĪŖ0.05NA | |
| D£® | ±ź×¼×“æöĻĀ£¬20gD2O·Ö×ÓÖŠĖłŗ¬ÖŠ×ÓŹżĪŖ10NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
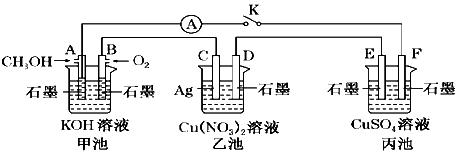
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com