
ij����С����ʵ�����Ʊ��������������йذ�������;�����ʵ�̽����
��1��д��ʵ������ȡ�����Ļ�ѧ����ʽ�� ��
��2���ϳɰ��Ի�ѧ������ҵ������Ҫ���塣д������������Ҫ��;��
�� �� ��
��3����С��ͬѧ�����ͼ��ʾװ��̽�������Ļ�ԭ�ԡ�
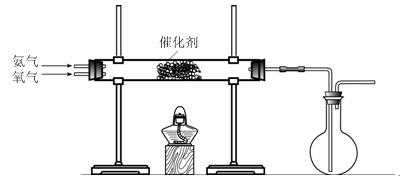
�ٰ��������Ļ�ѧ����ʽΪ ��
����ʵ��ʱͨ�백�������ʹ��죬����ƿ�л���ְ��̣������ʵĻ�ѧʽΪ ��
��4����С��ͬѧ�������Ͷ�����̼Ϊԭ���Ʊ������ϴ�����̼�����Һ������¼� ����������
�ټ������Ƚ�������̼ͨ��ˮ�У�����ܽ����ͨ�백����
�ҷ������Ƚ�����ͨ��ˮ�У�����ܽ����ͨ�������̼��
�����ķ����ǣ� �������� ��
�ڼ����������NH4+�ķ���Ϊ ��
���������ʾʽд����ˮ�д��ڵ�������� ��
��1��2NH4Cl��Ca(OH)2![]() 2NH3����CaCl2��2H2O ��1�֣�
2NH3����CaCl2��2H2O ��1�֣�
��2�� ���ƻ��� �������� ����2�֣�
��3���� 4NH3 + 5O2![]() 4NO + 6H2O ��NH4NO3 ����4�֣�
4NO + 6H2O ��NH4NO3 ����4�֣�
��4���ټ�������2�֣� ������Ӧ�յ�������ҷ�����Ӧ�յ���ѿ��� ��
������̼��ˮ���ܽ�Ƚ�С��������ˮ���ܽ�Ⱥܴ���ͨ�������̼����ͨ�백��������������� ��1�֣�
��ȡ������������Թ��У�����ŨNaOH��Һ�����ȣ����Թܿڷ���ʪ��ĺ�ɫʯ����ֽ������ֽ��������֤����������NH4+��(2��)
�� N��H��N O��H��O O��H��N N��H��O ��д��1��1�ֹ�4�֣�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �� |
| ��� |
| 2800�� |
| �� |
| C |
| ��ԭ |
| HCl |
| ||
| 714�� |
| HCl |
| ���ý��� |
| ��ԭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Լռ����������71%����ˮ��ѧ��Դ�����þ��зdz�������ǰ����
��1����ˮɹ�οɻ�ô��Σ���ʵ�����д��ξ����ܽ⡢ �� ���Ƶþ��Ρ�
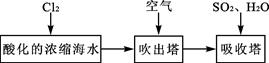
��2��þ����Ͻ���һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ģ�����Ҫ�������£�
��Ϊ��ʹMgSO4ת��ΪMg(OH) 2���Լ��ٿ���ѡ�� ��ҪʹMgSO4��ȫת��Ϊ�����������Լ��ٵ���Ӧ ��
���Լ��ڿ���ѡ�� ��
���Դӽ�Լ��Դ����߽���þ�Ĵ��ȷ������������˵�ұþ������ ��
A�� B��
C�� D��
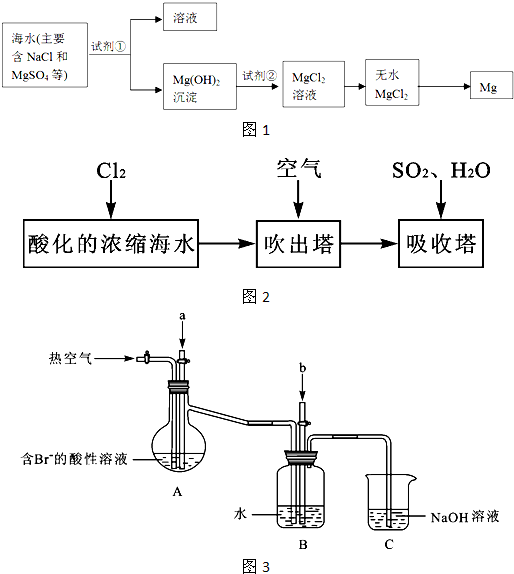
��3���弰�仯������;ʮ�ֹ㷺���ҹ����ڴ�����չ��ˮ������о��Ϳ�����������ҵ��Ũ����ˮΪԭ����ȡ��IJ��ֹ������£�
ij����С����ʵ����ģ�����������������װ�ý���ʵ�飨��������Ʒ���ѱ��������г�װ������ȥ����
��Aװ����ͨ��a�����Ŀ���ǣ������ӷ���ʽ��ʾ�� ��
��Aװ����ͨ��a����һ��ʱ���ֹͣͨ�룬��ͨ�ȿ�����ͨ���ȿ�����Ŀ����
��
�۷�Ӧ�����У�Bװ������SO42-���ɡ�����SO42-�ķ����� ��
��Cװ�õ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꼪��ʡ��ʦ���������и�һ��ѧ����ĩ���Ի�ѧ���� ���ͣ������
����Լռ����������71%����ˮ��ѧ��Դ�����þ��зdz�������ǰ����
��1����ˮɹ�οɻ�ô��Σ���ʵ�����д��ξ����ܽ⡢ �� ���Ƶþ��Ρ�
��2��þ����Ͻ���һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ģ�����Ҫ�������£�
��Ϊ��ʹMgSO4ת��ΪMg(OH) 2���Լ��ٿ���ѡ�� ��ҪʹMgSO4��ȫת��Ϊ�����������Լ��ٵ���Ӧ ��
���Լ��ڿ���ѡ�� ��
���Դӽ�Լ��Դ����߽���þ�Ĵ��ȷ������������˵�ұþ������ ��
A�� | B��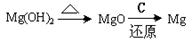 |
C�� | D�� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�걱���а�һ��ѧ��һ�ڶ�ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
����Լռ����������71%����ˮ��ѧ��Դ�����þ��зdz�������ǰ����
��1����ˮɹ�οɻ�ô��Σ���ʵ�����д��ξ����ܽ⡢ �� ���Ƶþ��Ρ�
��2��þ����Ͻ���һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ģ�����Ҫ�������£�
��Ϊ��ʹMgSO4ת��ΪMg(OH) 2���Լ��ٿ���ѡ�� ��ҪʹMgSO4��ȫת��Ϊ�����������Լ��ٵ���Ӧ ��
���Լ��ڿ���ѡ�� ��
���Դӽ�Լ��Դ����߽���þ�Ĵ��ȷ������������˵�ұþ������ ��
��3���弰�仯������;ʮ�ֹ㷺���ҹ����ڴ�����չ��ˮ������о��Ϳ�����������ҵ��Ũ����ˮΪԭ����ȡ��IJ��ֹ������£�
ij����С����ʵ����ģ�����������������װ�ý���ʵ�飨��������Ʒ���ѱ��������г�װ������ȥ����
��Aװ����ͨ��a�����Ŀ���ǣ������ӷ���ʽ��ʾ�� ��
��Aװ����ͨ��a����һ��ʱ���ֹͣͨ�룬��ͨ�ȿ�����ͨ���ȿ�����Ŀ����
��
�۷�Ӧ�����У�Bװ������SO42-���ɡ�����SO42-�ķ����� ��
��Cװ�õ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꼪��ʡ��һ��ѧ����ĩ���Ի�ѧ���� ���ͣ������
����Լռ����������71%����ˮ��ѧ��Դ�����þ��зdz�������ǰ����
��1����ˮɹ�οɻ�ô��Σ���ʵ�����д��ξ����ܽ⡢ �� ���Ƶþ��Ρ�
��2��þ����Ͻ���һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ģ�����Ҫ�������£�

��Ϊ��ʹMgSO4ת��ΪMg(OH) 2���Լ��ٿ���ѡ�� ��ҪʹMgSO4��ȫת��Ϊ�����������Լ��ٵ���Ӧ ��
���Լ��ڿ���ѡ�� ��
���Դӽ�Լ��Դ����߽���þ�Ĵ��ȷ������������˵�ұþ������ ��
A�� B��
B��
C��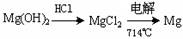 D��
D��
��3���弰�仯������;ʮ�ֹ㷺���ҹ����ڴ�����չ��ˮ������о��Ϳ�����������ҵ��Ũ����ˮΪԭ����ȡ��IJ��ֹ������£�

ij����С����ʵ����ģ�����������������װ�ý���ʵ�飨��������Ʒ���ѱ��������г�װ������ȥ����
��Aװ����ͨ��a�����Ŀ���ǣ������ӷ���ʽ��ʾ�� ��
��Aװ����ͨ��a����һ��ʱ���ֹͣͨ�룬��ͨ�ȿ�����ͨ���ȿ�����Ŀ����
��
�۷�Ӧ�����У�Bװ������SO42-���ɡ�����SO42-�ķ����� ��
��Cװ�õ������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com