
ĻĀĮŠ×Ŗ»Æ¹ŲĻµÖŠ£¬X”¢YŹĒÉś»īÖŠÓĆĶ¾¹ć·ŗµÄĮ½ÖÖ½šŹōµ„ÖŹ£¬A”¢BŹĒŃõ»ÆĪļ£¬A³Źŗģ×ŲÉ«£¬C”¢D”¢EŹĒ֊ѧ³£¼ūµÄČżÖÖ»ÆŗĻĪļ”£·ÖĪö×Ŗ»Æ¹ŲĻµ»Ų“šĪŹĢā£ŗ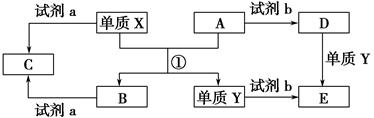
(1)ĒėŠ“³ö·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½_____________________________________”£
(2)¼ģŃéDČÜŅŗÖŠYĄė×ӵķ½·ØŹĒ__________________________________”£
(3)ČōŹŌ¼ĮaŹĒNaOHČÜŅŗ£¬Š“³öµ„ÖŹXÓėNaOHČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½______________________________”£
(4)ČōŹŌ¼ĮbŹĒH2SO4£¬¹¤ŅµÉĻÓĆE”¢H2SO4ŗĶNaNO2ĪŖŌĮĻÖĘČ”øߊ§¾»Ė®¼ĮY(OH)SO4£¬ŅŃÖŖ»¹Ō²śĪļĪŖNO£¬ŌņøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ________________________________________”£
(5)¹¤ŅµÉĻµē½āČŪČŚµÄBÖĘČ”XŹ±£¬ČōŃō¼«²śÉśµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ33.6 m3£¬ŌņŅõ¼«²śĪļµÄÖŹĮæĪŖ________kg”£
 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
¼×”¢ŅŅ”¢±ū”¢¶”ĖÄÖÖĪļÖŹÖŠ£¬¼×”¢ŅŅ”¢±ū¾łŗ¬ÓŠĻąĶ¬µÄijÖÖŌŖĖŲ£¬ĖüĆĒÖ®¼ä¾ßÓŠČēĻĀ×Ŗ»Æ¹ŲĻµ£ŗ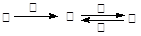 ”£ĻĀĮŠÓŠ¹ŲĪļÖŹµÄĶʶĻ²»ÕżČ·µÄŹĒ(”” ””)
”£ĻĀĮŠÓŠ¹ŲĪļÖŹµÄĶʶĻ²»ÕżČ·µÄŹĒ(”” ””)
| A£®Čō¼×ĪŖ½¹Ģ棬Ōņ¶”æÉÄÜŹĒO2 | B£®Čō¼×ĪŖSO2£¬Ōņ¶”æÉÄÜŹĒ°±Ė® |
| C£®Čō¼×ĪŖFe£¬Ōņ¶”æÉÄÜŹĒĀČĘų | D£®Čō¼×ĪŖNaOH ČÜŅŗ£¬Ōņ¶”æÉÄÜŹĒAlCl3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
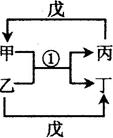
A~H¾łĪŖ¶ĢÖÜĘŚŌŖĖŲ£¬A~FŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĻą¶ŌĪ»ÖĆČēĶ¼1ĖłŹ¾£¬GÓėĘäĖüĘßÖÖŌŖĖŲ
| Ķ¼1 | |||
| A | B | C | |
| D | | E | F |
| Ķ¼2 | |||
 | |||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(12·Ö)ŅŃÖŖ½šŹōµ„ÖŹAŹĒÉś²śÉś»īÖŠÓĆĮæ×ī“óµÄ½šŹō”£ DŹĒÄŃČÜÓŚĖ®µÄ°×É«¹ĢĢ唣 FĪŖŗģŗÖÉ«¹ĢĢ唣ĪŽÉ«ĘųĢå¼×ÓöĘųĢå±ūĮ¢¼“Éś³Éŗģ×ŲÉ«µÄĘųĢåŅŅ”££ØĶ¼ÖŠ²æ·Ö²śĪļŗĶ·“Ó¦µÄĢõ¼žĀŌ£©”£
Ēėøł¾ŻŅŌÉĻŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öĻĀĮŠĪļÖŹµÄ»ÆѧŹ½C__________G__________”£
Š“³ö·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½________________________________ ”£
·“Ó¦¢ŚµÄĄė×Ó·½³ĢŹ½_____________________________”£
£Ø3£©·“Ó¦¢ÜÕūøö¹ż³Ģ¹Ū²ģµ½µÄĻÖĻóĪŖ_____________________________”£
£Ø4£©·“Ó¦¢ŻÖŠ£¬Čō½«³äĀśĘųĢåŅŅµÄŹŌ¹Üµ¹æŪŌŚĖ®²ŪÖŠ£¬³ä·Ö·“Ó¦ŗó£¬ŹŌ¹ÜÄŚŅŗĢåÕ¼ŹŌ¹Ü×ÜĢå»ż_______________”£
£Ø5£©Ä³ÖÖʶŃŖÖ¢»¼ÕßÓ¦²¹³äCĪļÖŹµÄŃōĄė×Ó”£ŗ¬øĆĄė×ÓµÄŅ©Ę¬Ķā±ķ°üÓŠŅ»²ćĢŲŹāµÄĢĒŅĀ£¬Õā²ćĢĒŅĀµÄ×÷ÓĆ¾ĶŹĒ±£»¤øĆĄė×Ó²»±»æÕĘųÖŠµÄŃõĘųŃõ»Æ”£ĪŖ¼ģŃé³¤ĘŚ·ÅÖƵÄŅ©Ę¬ŅŃ¾Ź§Š§£¬½«Ņ©Ę¬Č„³żĢĒŅĀŗóŃŠĖé£¬Č”ÉŁĮæŃŠĖéµÄŅ©Ę¬·ÅČėÉÕ±ÖŠ£¬¼ÓŹŹĮæµÄÕōĮóĖ®£¬Č»ŗóµĪ¼ÓŹżµĪ__________ČÜŅŗ£¬ČÜŅŗĻŌ__________É«£¬±ķĆ÷øĆŅ©Ę¬ŅŃŹ§Š§”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻĀĶ¼ŹĒĪŽ»śĪļA”«MŌŚŅ»¶ØĢõ¼žĻĀµÄ×Ŗ»Æ¹ŲĻµ£Ø²æ·Ö²śĪļ¼°·“Ó¦Ģõ¼žĪ“ĮŠ³ö£©”£ĘäÖŠ£¬IŹĒÓɵŚČżÖÜĘŚŌŖĖŲ×é³ÉµÄµ„ÖŹÖŠČŪµć×īøߵĽšŹō£¬KŹĒŅ»ÖÖŗģ×ŲÉ«ĘųĢ唣
ĢįŹ¾£ŗ4FeS2£«11O2øßĪĀ,2Fe2O3£«8SO2
ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
£Ø1£©ŌŚÖÜĘŚ±ķÖŠ£¬×é³Éµ„ÖŹGµÄŌŖĖŲĪ»ÓŚµŚ________ÖÜĘŚ________×唣
£Ø2£©ŌŚ·“Ó¦¢ßÖŠŃõ»Æ¼ĮÓė»¹Ō¼ĮµÄĪļÖŹµÄĮæÖ®±ČĪŖ________”£
£Ø3£©ŌŚ¢Ś”¢¢Ū”¢¢Ž”¢¢įÖŠ¼ČŹōÓŚ»ÆŗĻ·“Ó¦ÓÖŹōÓŚ·ĒŃõ»Æ»¹Ō·“Ó¦µÄŹĒ________£ØĢīŠņŗÅ£©”£
£Ø4£©·“Ó¦¢ÜµÄĄė×Ó·½³ĢŹ½ŹĒ_____________________________________”£
£Ø5£©½«»ÆŗĻĪļDÓėKNO3”¢KOH¹²ČŪ£¬æÉÖʵĆŅ»ÖÖ”°ĀĢÉ«”±»·±£øߊ§¾»Ė®¼ĮK2FeO4£ØøßĢśĖį¼Ų£©£¬Ķ¬Ź±»¹Éś³ÉKNO2ŗĶH2O”£øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¢ń.ĻĀĮŠ»ÆŗĻĪļÖŠ£¬ŗ¬ÓŠ·Ē¼«ŠŌ¹²¼Ū¼üµÄĄė×Ó»ÆŗĻĪļŹĒ (””””)”£
| A£®CaC2 | B£®N2H4 | C£®Na2S2 | D£®NH4NO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŌŖĖŲ¼°Ęä»ÆŗĻĪļŌŚÉś²ś”¢Éś»īÖŠ¾ßÓŠ¹ć·ŗµÄÓĆĶ¾”£
¢ń.øõ»Æѧ·įø»¶ą²Ź.
£Ø1£©ŌŚ³£ĪĀĻĀ£¬øõÄÜ»ŗĀżÓėĻ”ĮņĖį·“Ó¦£¬Éś³ÉĄ¶É«ČÜŅŗ”£ ÓėĶĻą±Č£¬Ę佚Źō»īĘĆŠŌ £ØĢī”°Ēæ”±»ņ”°Čõ”±£©£»
¢ĘCr( OH)3ŗĶAl( OH)3ĄąĖĘ£¬Ņ²ŹĒĮ½ŠŌĒāŃõ»ÆĪļ£¬ŌŚĖ®ÖŠ“ęŌŚĖįŹ½ŗĶ¼īŹ½µēĄėĘ½ŗā£¬ĘäĖįŹ½µēĄė·½³ĢŹ½ŹĒ £»
¢Ē¹¤ŅµÉĻ¾»»Æ“¦ĄķøõĪŪČ¾·½·ØÖ®Ņ»ŹĒ£ŗ½«ŗ¬K2Cr2O7ĖįŠŌ·ĻĖ®·ÅČĖµē½ā²ŪÄŚ£¬¼ÓČėŹŹĮæµÄNaCl£¬ŅŌFeŗĶŹÆÄ«ĪŖµē¼«½ųŠŠµē½ā”£¾¹żŅ»¶ĪŹ±¼äŗó£¬Éś³ÉCr(OH)3ŗĶFe(OH)3³Įµķ³żČ„(ŅŃÖŖKsP[ Fe(OH)3]=4.0”Į10-38£¬KsP[Cr(OH)3]=6.0”Įl0-31)”£ŅŃÖŖµē½āŗóµÄČÜŅŗÖŠc( Fe3+)ĪŖ2.0”Į10£13mol/L£¬ŌņČÜŅŗÖŠc(Cr3+)ĪŖ mol/L”£
¢ņ.ĪļÖŹA”«HÓŠČēĶ¼ĖłŹ¾×Ŗ»Æ¹ŲĻµ(²æ·ÖÉś³ÉĪļĪ“ĮŠ³ö)”£A”¢E”¢F”¢G¾łĪŖĘųĢ壬DĪŖ½šŹōµ„ÖŹ”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©AµÄµē×ÓŹ½ĪŖ DµÄ»ÆѧŹ½ £¬CČÜŅŗµÄĆū³ĘŹĒ ”£
£Ø2£©·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½ĪŖ £»
·“Ó¦¢ŪµÄĄė×Ó·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
A”¢B”¢C”¢D”¢E”¢FĮłÖÖĪļÖŹµÄĻą»„×Ŗ»Æ¹ŲĻµČēÓŅĶ¼ĖłŹ¾£Ø·“Ó¦Ģõ¼ž¼°²æ·Ö²śĪļĪ“ĮŠ³ö£©”£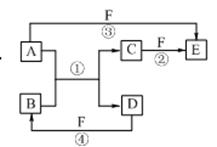
£Ø1£©ČōAŹĒ³£¼ū½šŹōµ„ÖŹ£¬ÓėBµÄĖ®ČÜŅŗ·“Ӧɜ³ÉCŗĶD”£D”¢FŹĒĘųĢåµ„ÖŹ£¬DŌŚFÖŠČ¼ÉÕŹ±·¢³ö²Ō°×É«»šŃę”£ŌņFĖł¶ŌÓ¦µÄŌŖĖŲŌŚÖÜĘŚ±ķĪ»ÖĆŹĒ £»·“Ó¦¢Ś£ØŌŚĖ®ČÜŅŗÖŠ½ųŠŠ£©µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø2£©ČōA”¢DĪŖ¶ĢÖÜĘŚŌŖĖŲ×é³ÉµÄ¹ĢĢåµ„ÖŹ£¬AĪŖ½šŹō£¬DĪŖ·Ē½šŹō”£ĒŅ¢Ū¢ÜĮ½øö·“Ó¦¶¼ÓŠŗģ×ŲÉ«ĘųĢåÉś³É£¬Ōņ·“Ó¦¢Ł”¢¢ÜµÄ»Æѧ·½³ĢŹ½·Ö±šĪŖ
¢Ł £»¢Ü ”£
£Ø3£©ČōA”¢D”¢F¶¼ŹĒ¶ĢÖÜĘŚ·Ē½šŹōµ„ÖŹ£¬ĒŅA”¢DĖłŗ¬ŌŖĖŲĶ¬Ö÷×壬A”¢FĖłŗ¬ŌŖĖŲĶ¬ÖÜĘŚ£¬CŹĒŅ»ÖÖÄÜÓėŃŖŗģµ°°×½įŗĻµÄÓŠ¶¾ĘųĢ壻ŌņĪļÖŹBµÄ¾§ĢåĄąŠĶŹĒ £¬·Ö×ÓEµÄ½į¹¹Ź½ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ČēĻĀĶ¼ŹĒ֊ѧ»Æѧ֊³£¼ūĪļÖŹÖ®¼äµÄŅ»Š©·“Ó¦¹ŲĻµ£¬ĘäÖŠ²æ·Ö²śĪļĪ“Š“³ö”£³£ĪĀĻĀXŗĶHŹĒ¹ĢĢ壬BŗĶGŹĒŅŗĢ壬ĘäÓą¾łĪŖĘųĢ壬 1 mol X·Ö½āµĆµ½A”¢B”¢Cø÷1 mol”£
ŹŌ»Ų“šĻĀĮŠø÷Ģā£ŗ
£Ø1£©Š“³öĻĀĮŠĪļÖŹµÄ»ÆѧŹ½£ŗX________£¬B________”£
£Ø2£©Š“³öĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
¢ŁH£«GØD”śA£«F£ŗ__________________________________________________________”£
¢ŚC£«DØD”śE£ŗ__________________________________________________________”£
£Ø3£©Š“³öĻĀĮŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
G£«CuØD”śE£ŗ___________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com