
�Ĵ��зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ��������ͼ��ʾ(ͼ��ijЩת�����輰������δ�г�)��
����д���пհף�
(1)��֪0.5 mol������0.5 molˮ������t �桢p kPaʱ����ȫ��Ӧ����һ��
��̼������(�ϳ���)��������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�______________________��
(2)�ںϳɰ���ʵ�����������У�����ȡ�Ĵ�ʩ֮һ�ǣ������ɵİ��ӻ�������м�ʱ����������������������ĵ���������ѭ�����ã�ͬʱ���䵪���������������û�ѧ��Ӧ���ʺͻ�ѧƽ��Ĺ۵�˵����ȡ�ô�ʩ�����ɣ�
________________________________________________________________��
(3)������ϳɰ�����ת����Ϊ75%ʱ����5.60��107 L����Ϊԭ���ܹ��ϳ�________L������(����������ڱ�״���²ⶨ)
(4)��֪���صĽṹ��ʽΪ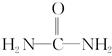 ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
��__________________�� ��_________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ο�����ͼ������֪E1��134 kJ��mol��1��E2��368 kJ��mol��1������Ҫ��ش����⣺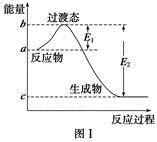
(1)ͼ����1 mol NO2(g)��1 mol CO(g)��Ӧ����CO2��NO�����е������仯ʾ��ͼ�����ڷ�Ӧ��ϵ�м����������Ӧ��������E1�ı仯�� (���������С�����䡱����ͬ)����H�ı仯�� ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ�� ��
(2)�״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧ���Ȼ�ѧ����ʽ���£�
��CH3OH(g)��H2O(g)===CO2(g)��3H2(g) ��H����49.0 kJ��mol��1
��CH3OH(g)��O2(g)===CO2(g)��2H2(g) ��H����192.9 kJ��mol��1
��֪��H2O(g)===H2O(l) ��H����44 kJ��mol��1����״�����ȼ��ΪҺ̬ˮ���Ȼ�ѧ����ʽΪ ��
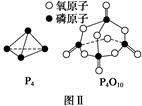
(3)�����ʾ�Dz��ֻ�ѧ���ļ��ܲ�����
| ��ѧ�� | P��P | P��O | O===O | P===O |
| ����/kJ��mol��1 | a | b | c | x |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���Ȼ���(TiCl4)����ȡ���캽�չ�ҵ���ϡ����ѺϽ����Ҫԭ�ϡ���������(��Ҫ�ɷ���FeTiO3)�Ʊ�TiCl4�Ȳ�Ʒ��һ�ֹ�������ʾ��ͼ���£�
(1)�����м�����м������Һ����ɫ����ʱ��Һ�Գ�ǿ���ԡ��ù����������·�Ӧ������
Fe��2Fe3��=3Fe2��
2TiO2��(��ɫ)��Fe��4H��=2Ti3��(��ɫ)��Fe2����2H2O
Ti3��(��ɫ)��Fe3����H2O=TiO2��(��ɫ)��Fe2����2H��
������������� ��
(2)�ڢڡ��۹��չ�������Ҫ�����������γ�TiO2��nH2O�ܽ������ܽ��ķ�ɢ�ʿ���ֱ����С�� ��Χ��
(3)���Ѣ����ƵõĹ���TiO2��nH2O������ϴ��ȥ���е����ʣ������Ƶ��Ѱۡ���֪25 ��ʱ��Ksp[Fe(OH)3]��2.79��10��39�����¶��·�ӦFe(OH)3��3H�� Fe3����3H2O��ƽ�ⳣ��K�� ��
Fe3����3H2O��ƽ�ⳣ��K�� ��
(4)��֪��TiO2(s)��2Cl2(g)=TiCl4(l)��O2(g) ��H����140 kJ��mol��1
2C(s)��O2(g)=2CO(g)����H����221 kJ��mol��1
д������TiO2�ͽ�̿��������Ӧ����Һ̬TiCl4��CO������Ȼ�ѧ����ʽ�� ��
(5)�������վ��гɱ��͡����õ�Ʒλ����Ϊԭ�ϵ��ŵ㡣������ɫ��ѧ����ù��������д��ڵIJ���֮���� (ֻҪ��д��һ��)��
(6)�����±���Ϣ��Ҫ���ƺ�����SiCl4���ʵ�TiCl4���ɲ��� ������
| | TiCl4 | SiCl4 |
| �۵�/�� | ��25.0 | ��68.6 |
| �е�/�� | 136.4 | 57.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ǰ����������һ��ȫ�����ӵ����⣬������������峣��������������CO2��
��Ⱦ������NOx��SOx�ȡ��������Щ����������þͿ��Գ�Ϊ��Ҫ����Դ���Ƚ���˶Ի�������Ⱦ���ֽ���˲�����ԴΣ�����⡣
(1)������̼�ǵ�������ЧӦ��������ף�Ŀǰ���Ǵ���������̼�ķ���֮һ��ʹ����������Ӧ�ϳɼ״����״�������ȼ�ϵ�ص���Ҫȼ�ϡ�CO2��H2��Ӧ�Ʊ�CH3OH��H2O�Ļ�ѧ����ʽΪ
(2)�ڸ�����һ����̼�ɽ���������ԭΪ������
��֪��
��C(s)��O2(g)=CO2(g)��H1����393.5 kJ��mol��1
��CO2(g)��C(s)=2CO(g)��H2����172.5 kJ��mol��1
��S(s)��O2(g)=SO2(g)��H3����296.0 kJ��mol��1
��д��CO��SO2��Ӧ���Ȼ�ѧ����ʽ ��
(3)���᳧���ô���ԭ��������β����CH4�ڴ������¿��Խ�NO2��ԭΪN2��
��֪��CH4(g)��2O2(g)=CO2(g)��2H2O(g)��H����889.6 kJ��mol��1��
N2(g)��2O2(g)=2NO2(g)��H����67.7 kJ��mol��1��
��CH4��ԭNO2����ˮ�����͵������Ȼ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪��ѧ����������ʽ���ܿ����ת������д�±��Ŀհ�:
| ��ѧ��Ӧ����ʽ(����) | ����ת����ʽ |
| �� | �ɻ�ѧ��ת��Ϊ���� |
��Pb+PbO2+2H2SO4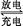 2PbSO4+2H2O 2PbSO4+2H2O | |
��CaCO3 CaO+CO2�� CaO+CO2�� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪���ٽ�úת��Ϊˮú������Ҫ��ѧ��ӦΪC(s)��H2O(g) CO(g)��H2(g)����C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
CO(g)��H2(g)����C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
C(s)��O2(g)=CO2 (g)����H����393.5 kJ��mol��1
CO(g)��1/2O2(g)=CO2(g)����H����283.0 kJ��mol��1
H2(g)��1/2O2(g)=H2O(g)����H����242.0 kJ��mol��1
��ش�
(1)����������Ϣ��д��CO��ˮ������Ӧ���Ȼ�ѧ����ʽ��____________________________��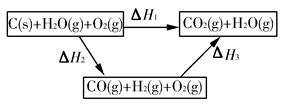
(2)��ͼ�Ǹ��ݸ�˹����������ѭ��ͼ������ͼ��ת����ϵ���Ȼ�ѧ����ʽ���㦤H3��________kJ/mol��
��ȽϦ�H1�릤H3��ֵ�Ƿ����˵����ˮú����ȼ��Ҫ��ֱ��ȼú�ų���������________(�ǻ��)ԭ����___________________________________��
(3)Ŀǰú�����仹��Ҫ����·�����·���䣬��������ѧ֪ʶ���������������·��·����ķ�����____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)�״���һ����Ҫ�Ļ�����Ʒ�������ü״��������Ʊ���ȩ����ȩ����̬�״�ת����������ϵ��ͼ��ʾ��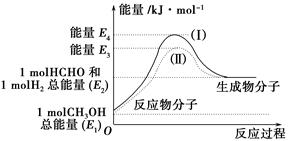
��Ӧ�����е�������ϵ
�ټ״�������ת��Ϊ��ȩ�ķ�Ӧ��________(����ȡ����ȡ�)��Ӧ��
�ڹ��̢�����̢�ķ�Ӧ���Ƿ���ͬ��____________ԭ����____________ ______________________________��
��д���״�������ת��Ϊ��ȩ���Ȼ�ѧ��Ӧ����ʽ______________ _____________________��
(2)��֪����CH3OH(g)��H2O(g)=CO2(g)��3H2(g)����H����49.0 kJ��mol��1
��CH3OH(g)��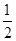 O2(g)=CO2(g)��2H2(g)����H����192.9 kJ��mol��1
O2(g)=CO2(g)��2H2(g)����H����192.9 kJ��mol��1
����˵����ȷ����________��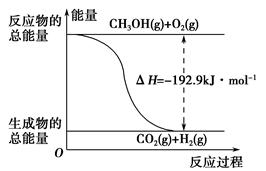
| A��CH3OHת���H2�Ĺ���һ��Ҫ�������� |
| B���ٷ�Ӧ�У���Ӧ�������������������������� |
C�����ݢ���֪��Ӧ��CH3OH(l)��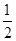 O2(g)=CO2(g)��2H2(g)�Ħ�H����192.9 kJ��mol��1 O2(g)=CO2(g)��2H2(g)�Ħ�H����192.9 kJ��mol��1 |
| D����Ӧ�ڵ������仯��ͼ��ʾ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��25 �桢101 kPa�������£�����1 mol H��H������436 kJ����������1 mol Cl��Cl������243 kJ�������γ�1 mol H��Cl���ų�431 kJ������H2��Cl2===2HCl�Ļ�ѧ��Ӧ������ͼ��ʾ��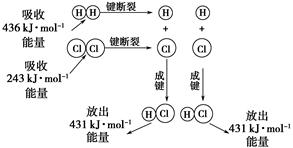
��ش������й����⣺
(1)��Ӧ��ϼ����յ�������Ϊ________��
(2)������ɼ��ų���������Ϊ________��
(3)�ж�H2��Cl2===2HCl��________(����ա��ų���)������
(4)��Ӧ���������________(�����������������)���������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)�״�����Ϊȼ�ϵ�ص�ԭ�ϡ���CH4��H2OΪԭ��,ͨ�����з�Ӧ���Ʊ��״���
��:CH4 (g)+H2O(g)=CO(g)+3H2(g) ��H="+206.0" kJ��mol-1
��:CO(g)+2H2(g)=CH3OH(g) ��H="-129.0" kJ��mol-1
CH4(g)��H2O(g)��Ӧ����CH3OH (g)��H2(g)���Ȼ�ѧ����ʽΪ ��
(2)�״���ˮ�ʻ����һ������Ⱦ,��һ�ֵ绯ѧ��������������Ⱦ,��ԭ����:ͨ���,��Co2+������Co3+,Ȼ����Co3+����������ˮ�еļ״�������CO2��������ʵ��������ͼװ��ʵ����������: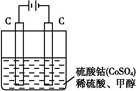
��д�������缫��Ӧʽ: ��
��д����ȥ�״������ӷ���ʽ: ��
(3)д����NaHCO3��ҺΪ���ʵ�Al������ԭ��صĸ�����Ӧʽ: ��
(4)�˹�����ɲ��ü�ӵ绯ѧ������ȥ��л�����е�����,ԭ����ͼ: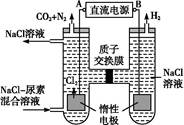
�ٵ�Դ�ĸ���Ϊ (�A����B��)��
���������з����ķ�Ӧ����Ϊ ��
�۵�������,��������Һ��pH����ǰ��Ƚ� ;���������ռ�������13.44 L(��״��),���ȥ������Ϊ g (����������ܽ�)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com