
(��10��)
������ȩͨ��Ϊ40�����ҵ���ȩ��Һ�����õ���ȩ��Һ������ֲ������ϲ�Ϊ��ɫ��״Һ�壬�²�Ϊˮ��Һ���ݲⶨ���ϲ�����Ϊ��ȩ�ļӺ�����(C2H4O)n�����ķе��ˮ�ķе�ߣ���������ȩ������ȩ����Һ���ױ�������Ϊ�ӱ��ʵ���ȩ��Һ����ȡ��ȩ(�Եõ���Һ)�����������·�Ӧԭ����
(1)(1.5��)�Ȱѻ�������õ�(C2H4O)n�������������Һ©�����ֲ��������������_____________��
(2) (1.5��)֤���Ƿ����в�����ȩ��������ʵ�������������_______��
(3) (2��)����������ȩ��Һ����Ũ�����У����ɺ�ɫ���ʡ����û�ѧ����ʽ��ʾ��һ���̣� . ��
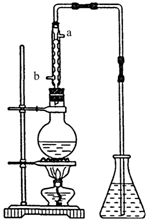 (4) (4��)��ȡ��ȩ��װ������ͼ����ƿ�зŵ���(C2H4O)n��6mol/L H2SO4�Ļ��Һ����ƿ�з�����ˮ�����������Һ���ڣ�(C2H4O)n�����ֽ⣬���ɵ����嵼����ƿ��ˮ�С�
(4) (4��)��ȡ��ȩ��װ������ͼ����ƿ�зŵ���(C2H4O)n��6mol/L H2SO4�Ļ��Һ����ƿ�з�����ˮ�����������Һ���ڣ�(C2H4O)n�����ֽ⣬���ɵ����嵼����ƿ��ˮ�С�
�� �������ܵ�Ŀ���� ������ˮ�Ľ����� ��(�a����b��)��
�� ��ƿ�ڵ��ܿڳ��ֵ����ݴ���������Һ��Ĺ����У����Խ��ԽС��ֱ����ȫ��ʧ��������˵���Ҵ��ĺ����������ʣ�
���۲쵽�����е�������Сʱ����Ҫ�IJ����ǣ�
�� ��n��3����(C2H4O)n�Ľṹ��ʽ�� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)�Ȱѻ�������õ�(C2H4O)n�������������Һ©�����ֲ��������������________��
(2)֤���Ƿ����в�����ȩ��������ʵ�������������________________________��
(3)����������ȩ��Һ����Ũ�����У����ɺ�ɫ���ʡ����û�ѧ����ʽ��ʾ��һ���̣�________________________________________________________________��
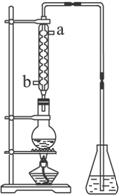
(4)��ȡ��ȩ��װ������ͼ����ƿ�зŵ���(C2H4O)n��6 mol��L-1 H2SO4�Ļ��Һ����ƿ������ˮ�����������Һ���ڣ�(C2H4O)n�����ֽ⣬���ɵ����嵼����ƿ�е�ˮ�С�
���������ܵ�Ŀ����________������ˮ�Ľ�����________��(�a����b��)����ƿ�ڵ��ܿڳ��ֵ����ݴ���������Һ��Ĺ����У����Խ��ԽС��ֱ����ȫ��ʧ����һ����˵���Ҵ��ĺ����������ʣ�________�����۲쵽�����е������Ѻ�Сʱ����Ҫ�IJ�����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�����д�����ѧ������һ��Ϳ��ѵ����ѧ�Ծ����������� ���ͣ�ʵ����
ˮ��ʯ��ѧʽΪ��[Mg6Al2(OH)16CO3]��4H2O����Է�������Ϊ602�����ǻ�������ȼ����������ʱ����4�ֲ�ͬ���������д�������ʷֽ�Ļ�ѧ����ʽ�� ��
��ҽҩ�ϣ�ˮ��ʯ������Ϊ����ҩ��������θ��ʮ��ָ������ȣ�ij�о���ѧϰС��Ҫ�ⶨһ�����۵�ˮ��ʯҩƬ��ˮ��ʯ������������
���������ϡ�ˮ��ʯ��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ��
[Mg6Al2(OH)16CO3]��4H2O+9H2SO4==6MgSO4+Al2(SO4)3+CO2��+21H2O��ҩƬ�г���ˮ��ʯ�⺬��һ�����ĸ��ϡ������۵����ʡ�
��������롿С���뽫��ҩƬ���ڿ����г��������ɲⶨ��С����ͨ��ˮ��ʯ�����ᷴӦԭ������ɲⶨ���������̽����
����Ʒ�������������˲�ͬ��ʵ�鷽����
С���ķ�������ȡ10.0g���۵�ˮ��ʯҩƬ��ĥ�ɷ�ĩ����ͨ����У�����������������ټ��٣��ٳ���ʣ����������Ϊ6.1g�����ٵ�������Ϊ������̼��ˮ����������������ˮ��ʯҩƬ��ˮ��ʯ����������Ϊ %��
С���ķ�����
��1��С���������ͼ��ʾ��ʵ��װ�á�ȡ10.0g���۵�ˮ��ʯҩƬ��ĥ�ɷ�ĩ������ʵ�顣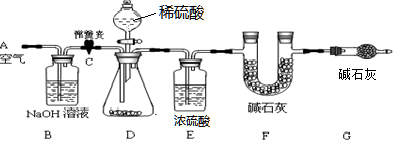
��˵������ʯ���������ƺ��������ƹ���Ļ���Bװ���з����Ļ�ѧ����ʽΪ ��
��2����������
�����Ӻ�װ�ã����װ�õ������Ԣڴ��ɼ�C����A������ͨ��һ��ʱ��Ŀ����۳���F�������ܹرյ��ɼ�C�������μ�ϡ������������ֱ��D��������ð���ݴ��ɼ�C���ٴλ���ͨ��һ��ʱ��������ٴγ���F����������ǰ������������Ϊ0.44g��
��3������̽��
B��Eװ�õ����÷ֱ��� �� ����û��Gװ�ã���ⶨ��ˮ��ʯ������������ ���ƫ����ƫС���������䡱����ʵ��ѡ��ϡ�������ѡ��ϡ����������� ��
��4�����ݼ���
����ʵ�����ݣ������ˮ��ʯҩƬ��ˮ��ʯ����������Ϊ %����д��������̣�
���������ۡ�������ʵ�鷽���У�����Ϊ������ʵ�鷽���� ����С����С��������һ��������������ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�����и�����һ��Ϳ��ѵ����ѧ�Ծ��������棩 ���ͣ�ʵ����
ˮ��ʯ��ѧʽΪ��[Mg6Al2(OH)16CO3]��4H2O����Է�������Ϊ602�����ǻ�������ȼ����������ʱ����4�ֲ�ͬ���������д�������ʷֽ�Ļ�ѧ����ʽ�� ��
��ҽҩ�ϣ�ˮ��ʯ������Ϊ����ҩ��������θ��ʮ��ָ������ȣ�ij�о���ѧϰС��Ҫ�ⶨһ�����۵�ˮ��ʯҩƬ��ˮ��ʯ������������
���������ϡ�ˮ��ʯ��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ��
[Mg6Al2(OH)16CO3]��4H2O+9H2SO4==6MgSO4+Al2(SO4)3+CO2��+21H2O��ҩƬ�г���ˮ��ʯ�⺬��һ�����ĸ��ϡ������۵����ʡ�
��������롿С���뽫��ҩƬ���ڿ����г��������ɲⶨ��С����ͨ��ˮ��ʯ�����ᷴӦԭ������ɲⶨ���������̽����
����Ʒ�������������˲�ͬ��ʵ�鷽����
С���ķ�������ȡ10.0g���۵�ˮ��ʯҩƬ��ĥ�ɷ�ĩ����ͨ����У�����������������ټ��٣��ٳ���ʣ����������Ϊ6.1g�����ٵ�������Ϊ������̼��ˮ����������������ˮ��ʯҩƬ��ˮ��ʯ����������Ϊ %��
С���ķ�����
��1��С���������ͼ��ʾ��ʵ��װ�á�ȡ10.0g���۵�ˮ��ʯҩƬ��ĥ�ɷ�ĩ������ʵ�顣
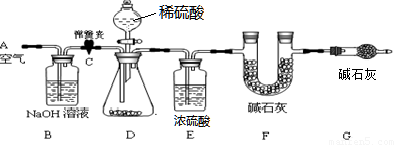
��˵������ʯ���������ƺ��������ƹ���Ļ���Bװ���з����Ļ�ѧ����ʽΪ ��
��2����������
�����Ӻ�װ�ã����װ�õ������Ԣڴ��ɼ�C����A������ͨ��һ��ʱ��Ŀ����۳���F�������ܹرյ��ɼ�C�������μ�ϡ������������ֱ��D��������ð���ݴ��ɼ�C���ٴλ���ͨ��һ��ʱ��������ٴγ���F����������ǰ������������Ϊ0.44g��
��3������̽��
B��Eװ�õ����÷ֱ��� �� ����û��Gװ�ã���ⶨ��ˮ��ʯ������������ ���ƫ����ƫС���������䡱����ʵ��ѡ��ϡ�������ѡ��ϡ����������� ��
��4�����ݼ���
����ʵ�����ݣ������ˮ��ʯҩƬ��ˮ��ʯ����������Ϊ %����д��������̣�
���������ۡ�������ʵ�鷽���У�����Ϊ������ʵ�鷽���� ����С����С��������һ��������������ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ�߶���ѧ�����п��Ի�ѧ���⣨�����棩 ���ͣ������
��ÿ��2�ֹ�10�֣�
I����1L������ͨ��CO2��H2��2mol����һ�������·�����Ӧ��CO2 + H2 CO + H2O��
CO + H2O��
�ش��������⣺
��1����830�������£���Ӧ�ﵽƽ��ʱCO2��ת����Ϊ50%��������ϵ�¶Ƚ���800�����ƽ�ⳣ��K1=0.81��������֪�÷�Ӧ������ӦΪ__________��Ӧ������ȡ��������ȡ�����
��2��800��ʱ��ijʱ�̲����ϵ�и����ʵ������£�n��CO2��=1.2mol��n��H2��=1.5mol��n��CO��=0.9mol��n��H2O��=0.9mol�����ʱ�÷�Ӧ ����.���������Ӧ�������淴Ӧ������ƽ��״̬������
II����һ�ݻ�Ϊ1L ���ܱ������м���һ������X��Y��������ѧ��ӦX(g)��2Y(s)  2Z(g)����H��0����ͼ��������X��Z�����ʵ���Ũ����ʱ��仯�����ߡ�
2Z(g)����H��0����ͼ��������X��Z�����ʵ���Ũ����ʱ��仯�����ߡ�

(1)0��10min �����������ѹǿ�� ___________������������С������ȷ������
(2)�Ʋ��ڵ�7minʱ���߱仯��ԭ������� ___��13minʱ���߱仯��ԭ������� __������ţ�
������Z���� ������X���� ������ �ܽ��� ��ʹ�ô���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com