
 �ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ�����壮������ѧ֪ʶ�ش��������⣺
�ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ�����壮������ѧ֪ʶ�ش��������⣺���� ��1��A��a��b����������Ӧ���У�Ũ��Խ��Ӧ����Խ�죻
B��c�㷴Ӧ������������ʵ������ڱ仯��
C��d���e�㶼����ƽ��״̬��n��N2�����䣻
D���÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ���¶�����ƽ�����淴Ӧ�ƶ���
��2������ɳ����ԭ��������ı�Ӱ��ƽ���һ����������Ũ�ȡ�ѹǿ���¶ȵȣ���ƽ������ܹ��������ָı�ķ����ƶ�����ɳ����ԭ�����õĶ���Ӧ���ڿ�����̣������������أ���ƽ���ƶ��أ���������ɳ����ԭ�����ͣ�
�ڼ���N2����ʼŨ��Ϊ$\frac{2mol}{2L}$=1mol/L��H2����ʼŨ��Ϊ$\frac{6mol}{2L}$=3mol/L��ƽ��ʱ����Ũ�ȱ仯��Ϊ����mol/L����
N2��g��+3H2��g���T2NH3��g��
��ʼŨ�ȣ�mol/L����1 3 0
�仯Ũ�ȣ�mol/L�������� 3���� 2����
ƽ��Ũ�ȣ�mol/L����1-���� 3��1-������ 2����
�ٸ���K=$\frac{{c}^{2}��N{H}_{3}��}{c��{N}_{2}����{c}^{3}��{H}_{2}��}$����ƽ�ⳣ����
��ЧΪ��ƽ��Ļ�����ѹǿ����һ��������ѹǿƽ�������ƶ���
��� �⣺��1��A��a��b����������Ӧ���У����ŷ�Ӧ�Ľ��У���Ӧ���Ũ����С����Ӧ������С����a�������Ӧ���ʱ�b���A��ȷ��
B��c�㷴Ӧ������������ʵ������ڱ仯��û�дﵽƽ��״̬����B����
C��d���e�㶼����ƽ��״̬��n��N2�����䣬d���e��n��N2����ȣ���C����
D���÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ���¶�����ƽ�����淴Ӧ�ƶ������������ʵ�������D��ȷ��
�ʴ�Ϊ��AD��
��2����A�����ڳ�ѹ������ѹǿ����ѧƽ��������ƶ��������ڰ����ĺϳɣ�������ɳ����ԭ�����ͣ���A��ѡ��
B��500��ĸ��£��������ڰ����ĺϳɣ����ǿ�����ߴ����Ĵ����ԣ���������ɳ����ԭ�����ͣ���Bѡ��
C������ý����������������ѧƽ����ƶ�����������ɳ����ԭ�����ͣ���Cѡ��
D�������ɵİ�Һ������ʱ����ϵ�з��������δ��Ӧ��N2��H2ѭ�����ϳ����У�����ʹ�û�ѧƽ�������ƶ��������ڰ��ĺϳɣ�������ɳ����ԭ�����ͣ���D��ѡ��
��ѡ��BC��
�ڼ���N2����ʼŨ��Ϊ$\frac{2mol}{2L}$=1mol/L��H2����ʼŨ��Ϊ$\frac{6mol}{2L}$=3mol/L��ƽ��ʱ����Ũ�ȱ仯��Ϊ����mol/L����
N2��g��+3H2��g���T2NH3��g��
��ʼŨ�ȣ�mol/L����1 3 0
�仯Ũ�ȣ�mol/L�������� 3���� 2����
ƽ��Ũ�ȣ�mol/L����1-���� 3��1-������ 2����
��ƽ�ⳣ��K=$\frac{{c}^{2}��N{H}_{3}��}{c��{N}_{2}����{c}^{3}��{H}_{2}��}$=$\frac{��2{��}_{��}��^{2}}{��1-{��}_{��}����[3��1-{��}_{��}��]^{3}}$=$\frac{4{{��}_{��}}^{2}}{27��1-{��}_{��}��^{4}}$��
��ЧΪ��ƽ��Ļ�����ѹǿ����һ��������ѹǿƽ�������ƶ���N2ת�������ʦ�����������
�ʴ�Ϊ��$\frac{4{{��}_{��}}^{2}}{27��1-{��}_{��}��^{4}}$������
���� ���⿼����ۺϣ��漰���ʵ�����Ũ����ʱ��ı仯���ߡ���ѧƽ���ƶ�ԭ������ѧƽ��ļ��㣬ע�ظ߿��������Ŀ��飬��Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NA��N2������NA��CO���ӵ�������Ϊ1��1 | |
| B�� | ˮ��Ħ����������NA��ˮ���ӵ��������֮�� | |
| C�� | �ڳ��³�ѹ��11.2LN2���еķ�����Ϊ0.5NA | |
| D�� | 1mol•L-1NaCl��Һ�С�����NA��Na+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
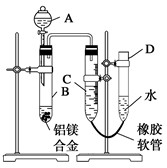 ijѧϰС������ͼ��ʾװ�òⶨ��þ�Ͻ������������������������ԭ��������
ijѧϰС������ͼ��ʾװ�òⶨ��þ�Ͻ������������������������ԭ���������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �屽��һ�ֳ��õĻ���ԭ�ϣ�ʵ�����Ʊ��屽��ʵ�鲽�����£�
�屽��һ�ֳ��õĻ���ԭ�ϣ�ʵ�����Ʊ��屽��ʵ�鲽�����£�| �� | �� | �屽 | |
| �ܶ�/g•cm-3 | 0.88 | 3.10 | 1.50 |
| �е�/�� | 80 | 59 | 156 |
| ��ˮ�е��ܽ�� | �� | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | һ������SO42- | B�� | һ������Ag+ | ||
| C�� | һ������SO42-��Ag+ | D�� | ���ܺ���SO42-��Ag+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com