
20.‘Ύ≥Θ―ΙΚΆ120Γφ ±Θ§‘ΎΟή±’»ίΤς÷–≥δ»κH2SΚΆO2ΒΡΜλΚœΤχΧεΙ≤100 mLΘ§”ΟΒγΜπΜ®Βψ»ΦΘ§Ψ≠≥δΖ÷Ζ¥”ΠΚσΘ§Μ÷Η¥ΒΫ‘≠Ή¥ΩωΘ§≤βΕ®»ίΤςΡΎ≤–ΝτΤχΧεΒΡΧεΜΐΓΘΨ≠≤βΕ®Θ§≤–ΝτΤχΧεΒΡΧεΜΐV1Υφ‘≠ΜλΚœΤχΧε÷–O2ΒΡΧεΜΐV2(O2)‘ωΦ”Εχ±δΜ·Θ§ΤδΙΊœΒ»γ”“ΆΦΥυ ΨΓΘ
 ΓΓΓΓΓΓΓΓΓΓΓΓ
ΓΓΓΓΓΓΓΓΓΓΓΓ
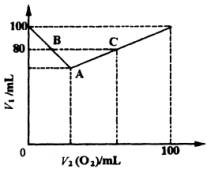
(1)Α―AΓΔBΓΔCΗςΒψΒΡ”–ΙΊ ΐΨίΧν»κœ¬±μΘΚ
|
|
A |
B |
C |
|
Ζ¥”Π«ΑΜλΚœΤχΧεΗς≥…Ζ÷ΧεΜΐ |
|
|
|
|
Ζ¥”ΠΚσ≤–ΝτΤχΧεΗς≥…Ζ÷ΧεΜΐ |
|
|
|
(2)Χ÷¬έV1ΚΆV2(O2)ΒΡΙΊœΒΘ§≤Δ”ΟΚ§V1ΚΆV2(O2)ΒΡΚ· ΐ Ϋ±μ Ψ÷°ΓΘ
(3)»τ≤–ΝτΤχΧεΧεΜΐV1=90mLΘ§‘≠ΜλΚœΤχΧεΒΡ≥…Ζ÷ «________Μρ________ΓΘ
 ΜΤΗ‘ΙΎΨϋΩΈΩΈΝΖœΒΝ–¥πΑΗ
ΜΤΗ‘ΙΎΨϋΩΈΩΈΝΖœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚΈοάμΫΧ―– “ Χβ–ΆΘΚ022
 ΓΓΓΓΓΓΓΓΓΓΓΓ
ΓΓΓΓΓΓΓΓΓΓΓΓ
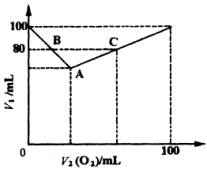
(1)Α―AΓΔBΓΔCΗςΒψΒΡ”–ΙΊ ΐΨίΧν»κœ¬±μΘΚ
|
|
A |
B |
C |
|
Ζ¥”Π«ΑΜλΚœΤχΧεΗς≥…Ζ÷ΧεΜΐ |
|
|
|
|
Ζ¥”ΠΚσ≤–ΝτΤχΧεΗς≥…Ζ÷ΧεΜΐ |
|
|
|
(2)Χ÷¬έV1ΚΆV2(O2)ΒΡΙΊœΒΘ§≤Δ”ΟΚ§V1ΚΆV2(O2)ΒΡΚ· ΐ Ϋ±μ Ψ÷°ΓΘ
(3)»τ≤–ΝτΤχΧεΧεΜΐV1=90mLΘ§‘≠ΜλΚœΤχΧεΒΡ≥…Ζ÷ «________Μρ________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ÷Σ ΕΨΪΫ≤”κΡήΝΠ―ΒΝΖΓΓΓΓΗΏ»ΐΜ·―ß Χβ–ΆΘΚ038
‘Ύ≥Θ―ΙΚΆ120Γφ ±Θ§‘ΎΟή±’»ίΤς÷–≥δ»κH2SΚΆO2ΒΡΜλΚœΤχΧεΙ≤100mLΘ§”ΟΒγΜπΜ®Βψ»ΦΘ§Ψ≠≥δΖ÷Ζ¥”ΠΚσΘ§Μ÷Η¥ΒΫ‘≠Ή¥ΩωΘ§≤βΕ®»ίΤςΡΎ≤–ΝτΤχΧεΧεΜΐΘ§Ψ≠≤βΕ®Θ§≤–ΝτΤχΧεΧεΜΐVΥφ‘≠ΜλΚœΤχΧε÷–O2ΒΡΧεΜΐ![]() ‘ωΦ”Εχ±δΜ·Θ§ΤδΙΊœΒ»γœ¬ΆΦΥυ ΨΘ°
‘ωΦ”Εχ±δΜ·Θ§ΤδΙΊœΒ»γœ¬ΆΦΥυ ΨΘ°
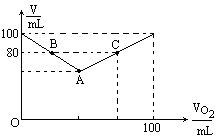
(1)ΫΪΆΦ÷–AΓΔBΓΔCΗςΒψΒΡ ΐΨίΧν»κœ¬±μΘ°

(2)Χ÷¬έVΚΆ![]() ΒΡΙΊœΒ≤Δ”ΟΚ§VΚΆ
ΒΡΙΊœΒ≤Δ”ΟΚ§VΚΆ![]() ΒΡΚ· ΐ Ϋ±μ ΨΘ°
ΒΡΚ· ΐ Ϋ±μ ΨΘ°
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013ΫλΗ Υύ ΓΗ Ι»“Μ÷–ΗΏ»ΐ…œ―ßΤΎΒΎ“Μ¥ΈΦλ≤βΩΦ ‘άμΉέΜ·―ß ‘ΨμΘ®¥χΫβΈωΘ© Χβ–ΆΘΚΧνΩ’Χβ
Θ®14Ζ÷Θ©Υ°ΟΚΤχ «“Μ÷÷ΗΏ–ßΤχΧε»ΦΝœΘ§Τδ÷ς“Σ≥…Ζ÷ «COΚΆH2Θ§Ω…”ΟΥ°’τΤχΆ®Ιΐ≥ψ»»ΒΡΧΩ÷ΤΒΟΘΚC (s) + H2O(g) CO (g) +H2 (g) ΓςHΘΫ+131kJ?molΘ≠1
CO (g) +H2 (g) ΓςHΘΫ+131kJ?molΘ≠1
Δ≈TΈ¬Ε»œ¬Θ§ΥΡΗω»ίΤς÷–ΨυΫχ––Ή≈…œ ωΖ¥”ΠΘ§Ης»ίΤς÷–ΧΩΉψΝΩΘ§ΤδΥϋΈο÷ ΒΡΈο÷ ΒΡΝΩ≈®Ε»ΦΑ’ΐΡφΖ¥”ΠΥΌ¬ ΙΊœΒ»γœ¬±μΥυ ΨΓΘ«κΧν–¥±μ÷–œύ”ΠΒΡΩ’ΗώΓΘ
| »ίΤς ±ύΚ≈ | c(H2O) /molΓΛLΘ≠1 | c(CO) /molΓΛLΘ≠1 | c(H2) /molΓΛLΘ≠1 | v’ΐΓΔvΡφ±»Ϋœ |
| I | 0.06 | 0.60 | 0.10 | v’ΐΘΫvΡφ |
| II | 0.06 | 0.50 | 0.40 | ΔΌ |
| III | 0.12 | 0.40 | 0.80 | v’ΐΘΦvΡφ |
| IV | 0.12 | 0.30 | ΔΎ | v’ΐΘΫvΡφ |
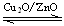 CH3OH(g) ΓςHΘΨ0ΗχΚœ≥…ΦΉ¥ΦΖ¥”ΠΧεœΒ÷–Ά®»κ…ΌΝΩCO‘ρΤΫΚβœρ “ΤΕ·Θ§Φθ–Γ―Ι«Ω‘ρΤΫΚβœρ “ΤΕ·Θ§ΫΒΒΆΈ¬Ε»‘ρΤΫΚβœρ “ΤΕ·Θ®ΧνΉσΘ§”“Θ§≤ΜΘ©
CH3OH(g) ΓςHΘΨ0ΗχΚœ≥…ΦΉ¥ΦΖ¥”ΠΧεœΒ÷–Ά®»κ…ΌΝΩCO‘ρΤΫΚβœρ “ΤΕ·Θ§Φθ–Γ―Ι«Ω‘ρΤΫΚβœρ “ΤΕ·Θ§ΫΒΒΆΈ¬Ε»‘ρΤΫΚβœρ “ΤΕ·Θ®ΧνΉσΘ§”“Θ§≤ΜΘ©≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ0103 ‘¬ΩΦΧβ Χβ–ΆΘΚΒΞ―ΓΧβ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com