��֪A��B��C��DΪ���壬����A�ʻ���ɫ��D��������ˮ���γɵ���Һ��ʹ��̪��죮����֮���ת����ϵ��ͼ1��ʾ��
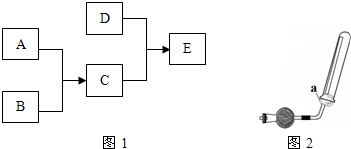
��1����B��ȼ���ѵ�������ʢ��A����ļ���ƿ�����Թ۲쵽��ʵ�������У�����ţ�
�٢ڢܢ�
�٢ڢܢ�
��
�ٷ��� �ڻ���ɫ��ȥ ��ƿ���а���
��ƿ���а��� �ݰ���ȼ�գ�������ɫ���� �ް���ȼ�գ�������ɫ����
��2��ʵ������D�Ļ�ѧ����ʽΪ
Ca��OH��
2+2NH
4Cl
CaCl
2+2NH
3��+2H
2O
Ca��OH��
2+2NH
4Cl
CaCl
2+2NH
3��+2H
2O
��
��3��ʵ���ҿ�����ͼ2��ʾװ���ռ�D������������ȷ��
�٢�
�٢�
������ţ���
��D���岻������ˮ���ռ� �ڸ������ʢ�м�ʯ�� ����ͼ�е�aΪ����ϡH
2SO
4����
��4����ҵ����D�Ļ�ѧ����ʽ��
��
��5������E�������ӵķ����ǣ�ȡ����E���Թ��У�
����ŨNaOH��Һ�����ȣ�������ɫ�д̼�����ζ�����壬��ʪ��ĺ�ɫʯ����ֽ���飬��ֽ��Ϊ��ɫ��֤��E����NH4+
����ŨNaOH��Һ�����ȣ�������ɫ�д̼�����ζ�����壬��ʪ��ĺ�ɫʯ����ֽ���飬��ֽ��Ϊ��ɫ��֤��E����NH4+
��


 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
 ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�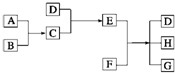 ��֪A��B��C��DΪ���壬E��FΪ���壬G���Ȼ��ƣ�����֮���ת����ϵ��ͼ��ʾ��?
��֪A��B��C��DΪ���壬E��FΪ���壬G���Ȼ��ƣ�����֮���ת����ϵ��ͼ��ʾ��?

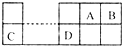 ��֪A��B��C��DΪ����������Ԫ�أ������λ�ù�ϵ��ͼ��C��B���γ����ӻ��� ��C3B2�����з�����ȷ���ǣ�������
��֪A��B��C��DΪ����������Ԫ�أ������λ�ù�ϵ��ͼ��C��B���γ����ӻ��� ��C3B2�����з�����ȷ���ǣ�������