
”¾ĢāÄæ”æI.H2AŌŚĖ®ÖŠ“ęŌŚŅŌĻĀĘ½ŗā£ŗH2A![]() H+ £«HA- £¬HA£
H+ £«HA- £¬HA£![]() H+£«A2- ”£
H+£«A2- ”£
£Ø1£©NaHAČÜŅŗĻŌĖįŠŌ£¬ŌņČÜŅŗÖŠĄė×ÓÅØ¶ČµÄ“óŠ”Ė³ŠņĪŖ____________________”£
£Ø2£©³£ĪĀŹ±£¬ČōĻņ0.1 mol/LµÄNaHAČÜŅŗÖŠÖšµĪµĪ¼Ó0.1mol/L KOHČÜŅŗÖĮČÜŅŗ³ŹÖŠŠŌ”£“ĖŹ±øĆ»ģŗĻČÜŅŗµÄĻĀĮŠ¹ŲĻµÖŠ£¬Ņ»¶ØÕżČ·µÄŹĒ_______________”£
A£®c(Na+ )£¾c(K+) B£®c(H +)c(OH)£½1”Į10-14
C£®c(Na+ )£½c(K+) D£®c(Na+ )£«c(K+ )£½c(HA£)£«c(A2- )
£Ø3£©ŅŃÖŖ³£ĪĀĻĀH2AµÄøĘŃĪ(CaA)±„ŗĶČÜŅŗÖŠ“ęŌŚŅŌĻĀĘ½ŗā£ŗCaA(s)![]() Ca2+ (aq)£«A2- (aq)£¬µĪ¼ÓÉŁĮæNa2A¹ĢĢ壬c(Ca2+ )_______________£ØĢī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±£©£¬ŌŅņŹĒ________________”£
Ca2+ (aq)£«A2- (aq)£¬µĪ¼ÓÉŁĮæNa2A¹ĢĢ壬c(Ca2+ )_______________£ØĢī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±£©£¬ŌŅņŹĒ________________”£
¢ņ.ŗ¬ÓŠCr2O72-µÄ·ĻĖ®¶¾ŠŌ½Ļ“ó£¬Ä³¹¤³§·ĻĖ®ÖŠŗ¬4.00”Į10-3 mol/LµÄCr2O72£”£ĪŖŹ¹·ĻĖ®ÄÜ“ļ±źÅÅ·Å£¬×÷ČēĻĀ“¦Ąķ£ŗ![]()
£Ø1£©øĆ·ĻĖ®ÖŠ¼ÓČėFeSO4”¤7H2OŗĶĻ”ĮņĖį£¬·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ_______________”£
£Ø2£©ÓūŹ¹25 LøĆ·ĻĖ®ÖŠCr2O7 ×Ŗ»ÆĪŖCr3+£¬ĄķĀŪÉĻŠčŅŖ¼ÓČė__________g FeSO4”¤7H2O”£
£Ø3£©Čō“¦ĄķŗóµÄ·ĻĖ®ÖŠ²ŠĮōµÄc(Fe)£½1”Į10-13mol/L£¬Ōņ²ŠĮōµÄ Cr3+ µÄÅضČĪŖ__________”££ØŅŃÖŖ£ŗKsp[Fe(OH)3]”Ö1.0”Į10-38mol/L£¬Ksp[Cr(OH)3]”Ö1.0”Į10-31 mol/L £©
”¾“š°ø”æI.£Ø1£©c(Na£«)£¾c(HA£)£¾c(H£«)£¾c(A2£)£¾c(OH£) £Ø2£©AB
£Ø3£©¼õŠ”£»¼ÓČėNa2A¹ĢĢ壬c(A2- )Ōö“󣬓Ӷųµ¼ÖĀČܽāĘ½ŗā×óŅĘ£¬c(Ca2+ )¼õŠ”
¢ņ.£Ø1£©Cr2O72££«6Fe2£«£«14H£«£½2Cr3£«£«6Fe3£«£«7H2O £Ø2£©166.8 £Ø3£©1.0”Į10£6mol/L
”¾½āĪö”æ
ŹŌĢāI.£Ø1£©H2AŌŚĖ®ÖŠ“ęŌŚŅŌĻĀĘ½ŗā£ŗH2A![]() H+ £«HA- £¬HA£
H+ £«HA- £¬HA£![]() H+£«A2- £¬ĖłŅŌNaHAÖ»“ęŌŚµēĄėĘ½ŗā£¬ČÜŅŗĻŌĖįŠŌ£¬ČÜŅŗÖŠĄė×ÓÅØ¶ČµÄ“óŠ”Ė³ŠņĪŖc(Na£«)£¾c(HA£)£¾c(H£«)£¾c(A2£)£¾c(OH£)”£
H+£«A2- £¬ĖłŅŌNaHAÖ»“ęŌŚµēĄėĘ½ŗā£¬ČÜŅŗĻŌĖįŠŌ£¬ČÜŅŗÖŠĄė×ÓÅØ¶ČµÄ“óŠ”Ė³ŠņĪŖc(Na£«)£¾c(HA£)£¾c(H£«)£¾c(A2£)£¾c(OH£)”£
£Ø2£©A”¢NaHAČÜŅŗ³ŹĖįŠŌ£¬Ļņ0.1mol/LµÄNaHAČÜŅŗÖŠÖšµĪµĪ¼Ó0.1mol/L KOHČÜŅŗÖĮČÜŅŗ³ŹÖŠŠŌŹ±£¬NaHAµÄĪļÖŹµÄĮæÓ¦“óÓŚĒāŃõ»Æ¼ŲµÄĪļÖŹµÄĮ棬ĖłŅŌĶ¬Ņ»»ģŗĻČÜŅŗÖŠc(Na+)£¾c(K+)£¬AÕżČ·£®B”¢Ė®µÄĄė×Ó»ż³£ŹżÓėĪĀ¶ČÓŠ¹Ų£¬ĪĀ¶ČŌ½øߣ¬Ė®µÄĄė×Ó»ż³£ŹżŌ½“󣬳£ĪĀĻĀĖ®µÄĄė×Ó»ż³£ŹżŹĒ10£14£¬BÕżČ·£»C”¢øł¾ŻAÖŠ·ÖĪöæÉÖŖC“ķĪó£»D”¢øł¾ŻµēŗÉŹŲŗćæÉÖŖc(Na+)£«c(K+)£«c(H+)£½c(HA£)£«2c(A2- )£¬Ōņc(H£«)£½c(OH£)£¬ĖłŅŌc£ØNa+£©+c£ØK+£©=c£ØHA-£©+2c£ØA2-£©£¬D“ķĪ󣬓š°øŃ”AB”£
£Ø3£©ÓÉÓŚ¼ÓČėNa2A¹ĢĢ壬c(A2- )Ōö“󣬓Ӷųµ¼ÖĀČܽāĘ½ŗā×óŅĘ£¬c(Ca2+ )¼õŠ””£
¢ņ.£Ø1£©ŃĒĢśĄė×Ó±»Ńõ»ÆµÄĄė×Ó·½³ĢŹ½ĪŖCr2O72££«6Fe2£«£«14H£«£½2Cr3£«£«6Fe3£«£«7H2O”£
£Ø2£©Ä³¹¤³§·ĻĖ®ÖŠŗ¬4.00”Į10-3 molL-1µÄCr2O72££¬n£ØCr2O72££©£½25L”Į4.00”Į10-3mol/L£½0.1mol£»ŅĄ¾ŻŃõ»Æ»¹Ō·“Ó¦Ąė×Ó·½³ĢŹ½Cr2O72££«6Fe2£«£«14H£«£½2Cr3£«£«6Fe3£«£«7H2O£¬µĆµ½n£ØFe2+£©£½0.6mol£»ŠčŅŖFeSO47H2OµÄÖŹĮæ=0.6mol”Į278g/mol£½166.8g£»
£Ø3£©Čō“¦ĄķŗóµÄ·ĻĖ®ÖŠ²ŠĮōµÄc£ØFe3+£©£½1”Į10-13molL-1£¬Ksp[Fe£ØOH£©3]£½c£ØFe3+£©”¤c3£ØOH-£©£½1.0”Į10-38£¬¼ĘĖćµĆµ½c3£ØOH-£©£½1”Į10-25mol/L£¬Ōņ²ŠĮōµÄCr3+µÄÅضČĪŖKsp[Cr£ØOH£©3]£½c£ØCr3+£©c3£ØOH-£©£½1.0”Į10-31 £¬c£ØCr3+£©£½1”Į10-6molL-1”£


 ÅąÓÅŗĆ¾ķµ„ŌŖ¼ÓĘŚÄ©¾ķĻµĮŠ“š°ø
ÅąÓÅŗĆ¾ķµ„ŌŖ¼ÓĘŚÄ©¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠ±ķŹ¾ĪļÖŹ½į¹¹µÄ»ÆѧÓĆÓļÕżČ·µÄŹĒ(””””)
A. ŅŅĻ©µÄ½į¹¹¼ņŹ½£ŗCH2CH2 B. Ņģ±ū»łµÄ½į¹¹¼ņŹ½£ŗ-CH(CH3)2
C. ōĒ»łµÄµē×ÓŹ½£ŗ ![]() D. ŠĀĪģĶéµÄ½į¹¹¼ņŹ½£ŗ
D. ŠĀĪģĶéµÄ½į¹¹¼ņŹ½£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æNO2”¢O2ŗĶČŪČŚKNO3æÉÖĘ×÷Č¼ĮĻµē³Ų£¬ĘäŌĄķČēĶ¼ĖłŹ¾”£øƵē³ŲŌŚ·Åµē¹ż³ĢÖŠŹÆÄ«¢ńµē¼«ÉĻÉś³ÉŃõ»ÆĪļY£¬YæÉŃ»·Ź¹ÓĆ”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
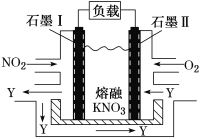
A. ·ÅµēŹ±£¬NO3-ĻņŹÆÄ«¢ņµē¼«ĒØŅĘ
B. ŹÆÄ«¢ņø½½ü·¢ÉśµÄ·“Ó¦ĪŖNO£«O2£«e£===NO3-
C. øƵē³Ų×Ü·“Ó¦Ź½ĪŖ4NO2£«O2===2N2O5
D. µ±ĶāµēĀ·Ķعż4 mol e£Ź±£¬øŗ¼«ÉĻ¹²²śÉś2mol N2O5
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŅŃÖŖNaClO2ŌŚĖ®ČÜŅŗÖŠÄÜ·¢ÉśĖ®½ā”£³£ĪĀŹ±£¬ÓŠ1 mol/LµÄHClO2ČÜŅŗŗĶ1mol/LµÄHBF4(·śÅšĖį)ČÜŅŗĘšŹ¼Ź±µÄĢå»ż¾łĪŖV0£¬·Ö±šĻņĮ½ČÜŅŗÖŠ¼ÓĖ®£¬Ļ”ŹĶŗóČÜŅŗµÄĢå»żĪŖV£¬ĖłµĆĒśĻßČēĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ
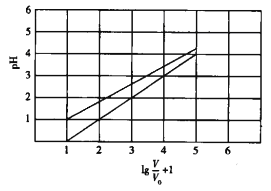
A. HClO2ĪŖČõĖį£¬HBF4ĪŖĒæĖį
B. ³£ĪĀĻĀHClO2µÄµēøßĘ½ŗā³£ŹżµÄŹżĮ漶ĪŖ10”Ŗ4
C. ŌŚ0”ÜpH”Ü5Ź±£¬HBF4ČÜŅŗĀś×ćpH=lg(V/V0)
D. 25”ꏱ1L pH=2µÄHBF4ČÜŅŗÓė100”ꏱ1L pH=2µÄHBF4ČÜŅŗĻūŗĵÄNaOHĻąĶ¬
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĪŖŃéÖ¤Ķ¬Ö÷×åŌŖĖŲŠŌÖŹµÄµŻ±ä¹ęĀÉ”£Ä³Š”×éÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆ½ųŠŠŹµŃé(¼Š³ÖŅĒĘ÷ŅŃĀŌČ„£¬×°ÖĆĘųĆÜŠŌŅŃ¼ģŃé)”£

ŹµŃé¹ż³Ģ£ŗ
¢ń.“ņæŖµÆ»É¼Š£¬“ņæŖ»īČūa£¬µĪ¼ÓÅØŃĪĖį”£
¢ņ.µ±×°ÖĆBŗĶ×°ÖĆCÖŠµÄČÜŅŗ¶¼±äĪŖ»ĘÉ«Ź±£¬¼Š½ōµÆ»É¼Š”£
¢ó.µ±×°ÖĆBÖŠČÜŅŗÓÉ»ĘÉ«±äĪŖ×ŲŗģÉ«Ź±£¬¹Ų±Õ»īČūa”£
¢ō.””
£Ø1£©½žÓŠNaOHČÜŅŗµÄĆŽ»ØµÄ×÷ÓĆ____________________________”£
£Ø2£©×°ÖĆAÖŠ·¢ÉśµÄÖĆ»»·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ___________________”£
£Ø3£©×°ÖĆBµÄČÜŅŗÖŠNaBrĶźČ«±»Ńõ»Æ£¬ŌņĻūŗÄCl2µÄĪļÖŹµÄĮæĪŖ__________”£
£Ø4£©ĪŖŃéÖ¤äåŌŖĖŲµÄ·Ē½šŹōŠŌĒæÓŚµāŌŖĖŲ£¬¹ż³Ģ¢ōµÄ²Ł×÷ŗĶĻÖĻóŹĒ__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ³£ĪĀĻĀĻņ20mL 0.1mol/L°±Ė®ÖŠĶØČėHClĘųĢ壬ČÜŅŗÖŠÓÉĖ®µēĄė³öµÄĒāĄė×ÓÅضČĖęĶØČėHClĘųĢåµÄĢå»ż±ä»ÆČēĶ¼ĖłŹ¾”£ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
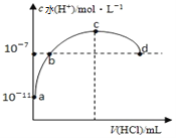
A. bµćĶØČėµÄHClĘųĢ壬ŌŚ±źæöĻĀĪŖ44.8mL
B. b”¢cÖ®¼äČÜŅŗÖŠc(NH4+)>c(Cl-)
C. Č”10mLµÄcµćČÜŅŗĻ”ŹĶŹ±£ŗc(NH4+)/c(NH3”¤H2O)¼õŠ”
D. dµćČÜŅŗ³ŹÖŠŠŌ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æijӊ»śĪļĶźČ«Č¼ÉÕÉś³ÉCO2ŗĶH2O”£½«12.4 gøĆÓŠ»śĪļµÄĶźČ«Č¼ÉÕ²śĪļĶعżÅØĮņĖį£¬ÅØĮņĖįŌöÖŲ10.8 g£¬ŌŁĶعż¼īŹÆ»Ņ£¬¼īŹÆ»ŅŌöÖŲĮĖ17.6 g”£ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ
A. øĆÓŠ»śĪļµÄ×ī¼ņŹ½ĪŖCH3O
B. øĆÓŠ»śĪļµÄ·Ö×ÓŹ½æÉÄÜĪŖCH3O
C. øĆÓŠ»śĪļµÄ·Ö×ÓŹ½Ņ»¶ØĪŖC2H6O2
D. øĆÓŠ»śĪļæÉÄÜŹōÓŚ“¼Ąą
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŗ£Ė®ÖŠäåŗ¬ĮæŌ¼ĪŖ 65 mg”¤L-1,“Óŗ£Ė®ÖŠĢįČ”äåµÄ¹¤ŅÕĮ÷³ĢČēĻĀ:

(1)ŅŌÉĻ²½Öč¢ńÖŠŅŃ»ńµĆÓĪĄėĢ¬µÄä壬²½Öč¢ņÓÖ½«Ö®×Ŗ±ä³É»ÆŗĻĢ¬µÄä壬ĘäÄæµÄŹĒ____________”£
(2)²½Öč¢ņĶØČėČČæÕĘų“µ³öBr2£¬ĄūÓĆĮĖäåµÄ____”£
A.Ńõ»ÆŠŌ ””B.»¹ŌŠŌ”” C.»Ó·¢ŠŌ D.øÆŹ“ŠŌ
(3)²½Öč¢ņÖŠÉę¼°µÄĄė×Ó·“Ó¦ČēĻĀ,ĒėŌŚĻĀĆę·½æņÄŚĢīČėŹŹµ±µÄ»Æѧ¼ĘĮæŹż:_____![]()
(4)ÉĻŹöĮ÷³ĢÖŠ“µ³öµÄäåÕōĘų£¬Ņ²æÉĻČÓƶžŃõ»ÆĮņĖ®ČÜŅŗĪüŹÕ£¬ŌŁÓĆĀČĘųŃõ»ÆŗóÕōĮ󔣊“³öäåÓė¶žŃõ»ÆĮņĖ®ČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½:__________”£
(5)ŹµŃéŹŅ·ÖĄėä廹æÉŅŌÓĆČܼĮŻĶČ”·Ø,ĻĀĮŠæÉŅŌÓĆ×÷äåµÄŻĶČ”¼ĮµÄŹĒ____”£
A.ŅŅ“¼ B.ĖÄĀČ»ÆĢ¼””””C.ÉÕ¼īČÜŅŗ D.±½
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æUO2ÓėÓĖµŖ»ÆĪļŹĒÖŲŅŖµÄŗĖČ¼ĮĻ£¬ŅŃÖŖ£ŗ3(NH4)4[UO2(CO3)3]![]() 3UO2+10NH3”ü+9CO2”ü+N2”ü+9H2O”ü
3UO2+10NH3ӟ+9CO2ӟ+N2ӟ+9H2Oӟ
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)»łĢ¬µŖŌ×Ó¼Ūµē×ÓÅŲ¼Ķ¼ĪŖ______”£
(2)·“Ó¦ĖłµĆĘųĢ¬»ÆŗĻĪļÖŠŹōÓŚ·Ē¼«ŠŌ·Ö×ӵďĒ_______(Ģī»ÆѧŹ½)”£
(3)ijÖÖÓĖµŖ»ÆĪļµÄ¾§Ģå½į¹¹ŹĒNaClŠĶ”£NaClµÄBom-HaberŃ»·ČēĶ¼ĖłŹ¾”£ŅŃÖŖ£ŗŌŖĖŲµÄŅ»øöĘųĢ¬Ō×Ó»ńµĆµē×Ó³ÉĪŖĘųĢ¬ŅõĄė×ÓŹ±Ėł·Å³öµÄÄÜĮæ³ĘĪŖµē×ÓĒ×ŗĶÄÜ”£ĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ________(Ģī±źŗÅ)”£
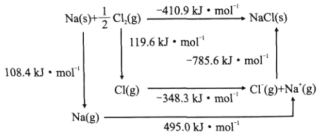
a.Cl-Cl¼üµÄ¼üÄÜĪŖ119.6kJ/mol b.NaµÄµŚŅ»µēĄėÄÜĪŖ603.4kJ/mol
c.NaClµÄ¾§øńÄÜĪŖ785.6kJ/mol d.ClµÄµŚŅ»µē×ÓĒ×ŗĶÄÜĪŖ348.3kJ/mol
(4)ŅĄ¾ŻVSEPRĄķĀŪĶĘ²āCO32-µÄæռ乹ŠĶĪŖ_________”£·Ö×ÓÖŠµÄ“óŲ¢¼üæÉÓĆ·ūŗÅŲ¢![]() ±ķŹ¾£¬ĘäÖŠm“ś±ķ²ĪÓėŠĪ³É“óŲ¢¼üµÄŌ×ÓŹż£¬n“ś±ķ²ĪÓėŠĪ³É“óŲ¢¼üµÄµē×ÓŹż(Čē±½·Ö×ÓÖŠµÄ“óŲ¢¼üæɱķŹ¾ĪŖŲ¢
±ķŹ¾£¬ĘäÖŠm“ś±ķ²ĪÓėŠĪ³É“óŲ¢¼üµÄŌ×ÓŹż£¬n“ś±ķ²ĪÓėŠĪ³É“óŲ¢¼üµÄµē×ÓŹż(Čē±½·Ö×ÓÖŠµÄ“óŲ¢¼üæɱķŹ¾ĪŖŲ¢![]() )£¬ŌņCO32-ÖŠµÄ“óŲ¢¼üÓ¦±ķŹ¾ĪŖ_____
)£¬ŌņCO32-ÖŠµÄ“óŲ¢¼üÓ¦±ķŹ¾ĪŖ_____
(5)UO2æÉÓĆÓŚÖʱøUF4£ŗ2UO2+5NH4HF2![]() 2UF4”¤2NH4F+3NH3”ü+4H2O£¬ĘäÖŠHF2µÄ½į¹¹±ķŹ¾ĪŖ[F”ŖH”F]-£¬·“Ó¦ÖŠ¶ĻĮѵĻÆѧ¼üÓŠ_______ (Ģī±źŗÅ)”£
2UF4”¤2NH4F+3NH3”ü+4H2O£¬ĘäÖŠHF2µÄ½į¹¹±ķŹ¾ĪŖ[F”ŖH”F]-£¬·“Ó¦ÖŠ¶ĻĮѵĻÆѧ¼üÓŠ_______ (Ģī±źŗÅ)”£
a.Ēā¼ü b.¼«ŠŌ¼ü c.Ąė×Ó¼ü d.½šŹō¼ü e.·Ē¼«ŠŌ¼ü
(6)ÓĖµŖ»ÆĪļµÄijĮ½ÖÖ¾§°ūČēĶ¼ĖłŹ¾£ŗ
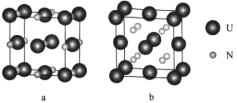
¢Ł¾§°ūaÖŠÓĖŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖ__________£¬ÓėU¾ąĄėĻąµČĒŅ×ī½üµÄUÓŠ_______øö”£
¢ŚŅŃÖŖ¾§°ūbµÄĆܶČĪŖdg/cm3£¬UŌ×ӵİė¾¶ĪŖr1cm£¬NŌ×ӵİė¾¶ĪŖĪŖr2cm£¬ÉčNAĪŖ°¢·ü¼ÓµĀĀŽ³£ŹżµÄÖµ£¬ŌņøĆ¾§°ūµÄæÕ¼äĄūÓĆĀŹĪŖ___________(ĮŠ³ö¼ĘĖćŹ½)”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com