
| A�� | ���£�0.1mol•L-1��CH3COOH��Һ�У�c��CH3COOH����c��CH3COO-�� | |
| B�� | 1L 0.1mol•L-1�ģ�NH4��2Fe��SO4��2��Һ�У�c��SO${\;}_{4}^{2-}$����c��NH${\;}_{4}^{+}$����c��Fe2+����c��H+����c��OH-�� | |
| C�� | ���£�0.1mol•L-1��CH3COONa��NaOH��Na2CO3������Һ��pH��С��˳��Ϊ��NaOH��CH3COONa��Na2CO3 | |
| D�� | ��0.01mol•L-1��NaHSO4��Һ�еμ�NaOH��Һ������ʱ��c��SO${\;}_{4}^{2-}$����c��Na+����c��OH-��=c��H+�� |
���� A��������ʵĵ����Ǽ������ģ�
B����NH4��2Fe��SO4��2��Һ�ж�Ԫǿ���ӵ�Ũ�ȣ���Ԫˮ���Ũ�ȣ�һԪǿ���ӵ�Ũ�ȣ�һԪˮ������ӵ�Ũ�ȣ��������ӣ��������ӣ�
C������ҺpH�������Һ�У��������ˮ��̶�Խ������ͬŨ�ȵ�������ҺpHԽ��
D����Һ�����ԣ����ߵ����ʵ�����ȣ�����c��Na+����c��SO42-����c��OH-��=c��H+����
��� �⣺A��������ʵĵ����Ǽ������ģ�����0.1mol•L-1��CH3COOH��Һ�У�c��CH3COOH����c��CH3COO-������A����
B����NH4��2Fe��SO4��2��Һ����Ũ�ȴ�С��˳��Ϊ��c��SO42-����c��NH4+����c��Fe2+����c��H+����c��OH-������B��ȷ��
C������ҺpH�������Һ�У��������ˮ��̶�Խ������ͬŨ�ȵ�������ҺpHԽ���������ˮ��̶�С��̼������ӣ�������������ҺpH��С˳���ǣ�CH3COONa��Na2CO3��NaOH����C����
D����Һ�����ԣ����ߵ����ʵ�����ȣ�����c��Na+����c��SO42-����c��OH-��=c��H+������D����
��ѡB��
���� ���⿼����pH��С���жϺ�����Ũ�ȴ�С�ıȽϣ���Ŀ�ѶȲ����ȸ�����Һ������δ�����࣬�ٸ��������ӵ�ˮ��Ũ���ж�pH��С��Ϊ�״��㣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
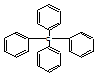
| A�� | �����ڱ���ͬϵ�� | B�� | ����������ԭ�ӹ�ƽ�� | ||
| C�� | ���ķ���ʽΪC25H24 | D�� | ����һ�ȴ���������ͬ���칹�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 8 | B�� | 9 | C�� | 11 | D�� | 12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
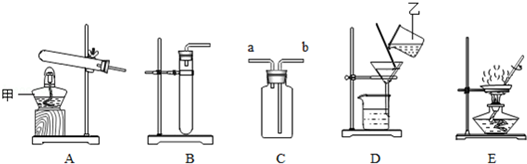
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������Һϡ��100����ϡ�ͺ���ҺpH��С˳�ۣ��ܣ��ڣ��� | |
| B�� | ������Ģۺֱܷ͢��õ�Ũ�ȵ�������Һ�кͣ�����������Һ��������ۣ��� | |
| C�� | ������Ģں͢ۻ������ǿ�������Σ���Ϻ���Һ������ | |
| D�� | ������Ģٺ͢ڷֱ���������п����Ӧ���ɵ�������ͬ��ͬѹ��������٣��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
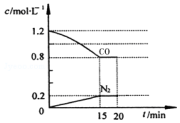 �ش��������⣺
�ش��������⣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com