
 ������ʵ������ļ��裬�������������Щ������һЩ�жϣ�
������ʵ������ļ��裬�������������Щ������һЩ�жϣ�| ʵ�鲽�� | ʵ������ | �� �� |
| ȡ��������ˮ | ����ֻ�õ���ɫ��Һ | һ��û��____________________________�� |
| �����а�ɫ���������ã��ϲ���Һ��ɫ | һ��û��___________________________�� | |
| ȡ�ڵĻ� ������� | ���ڳ����м������ᣬ���������ܽ⣻���ˣ�����Һ�м���AgNO3��Һ��ϡ���ᣬ�а�ɫ���� | һ������_____________________________�� �����м�����������ӷ���ʽ�� _______________________________________�� �ܷ�ȷ��ԭ�������Ƿ���NaCl����˵������ _______________________________________ _______________________________________�� |
| ʵ�鲽�� | ʵ������ | �� �� |
| ȡ���� ����ˮ | ����ֻ�õ���ɫ��Һ | һ��û��Ba(NO3)2��Na2CO3����1�֣� |
| �����а�ɫ���������ã��ϲ���Һ��ɫ | һ��û��CuSO4����1�֣� | |
| ȡ�ڵĻ� ������� | ���ڳ����м������ᣬ���������ܽ⣻���ˣ�����Һ�м���AgNO3��Һ��ϡ���ᣬ�а�ɫ���� | һ������Na2CO3 Na2SO4 Ba(NO3)2��2�֣� BaCO3��2H����Ba2����CO2����H2O��2�֣� ����ȷ������Ϊǰ�����������������Cl������2�֣� |


 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
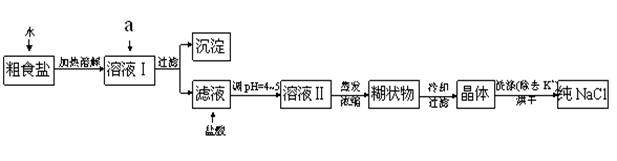
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���Ҵ������ᣩ��ʯ�ҹ��� |
| B���Ҵ�����ȩ��ͨ��ˮ���������� |
| C���������������ᣩ̼������Һ��Һ |
| D�����ᣨ��ȩ��ͨ��O2�����³�ѹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
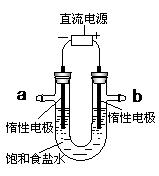
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������ᴿ�ܽ����ʱ��Ӧ���ˮ����ʹ��ҺϡЩ����ֹʳ���ܽⲻ��ȫ�� |
| B�������ᴿ��ȥ�����������Ժ���Һ���������ڼ���Ũ���� |
| C�������ᴿ��������ʣ������Һ��ʱ��ֹͣ���ȣ��������Ƚ�Һ�����ɡ� |
| D�����Ƶõľ���ת�Ƶ����ƹ��������ô���ˮ����ϴ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ܢ� | B���ܢ� | C���٢� | D���ܢ� |
�鿴�𰸺ͽ���>>
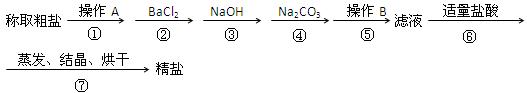
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

 ��
�� 
 ��3���ӻ��������ĽǶȿ��ǣ���
��3���ӻ��������ĽǶȿ��ǣ��� ��Ϊ��ʵ�����Ƹ���θĽ�?��д��һ�ָĽ�����:
��Ϊ��ʵ�����Ƹ���θĽ�?��д��һ�ָĽ�����:�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���Ҵ��е����ᣨ��ʯ�ң����� |
| B�������е���ϩ��NaOH��Һ��ϴ���� |
| C���屽�е��壨KI��Һ����Һ�� |
| D�����������е����ᣨ����NaOH��Һ�����ˣ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��1������۵�ʵ����������� �����������ʵ�������������� �������Ŀ���ǴӺ��ⱽ��Һ�з�������ʵ�ͻ��ձ����ò����ʵ����������� ��
��1������۵�ʵ����������� �����������ʵ�������������� �������Ŀ���ǴӺ��ⱽ��Һ�з�������ʵ�ͻ��ձ����ò����ʵ����������� ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com