

(1)1 molˮ����______________ mol���������
(2)ˮ���ӵ����������ֺ�����ͬ����������������뷽��ʽΪ______________________��
(3)�ڱ��Ľṹ�У�ÿ��ˮ���������ڵ�4��ˮ��������������ӣ��ڱ��г�����⣬�����ڷ��Ӽ�������(11 kJ��mol-1)����֪������������51 kJ��mol-1������������������______________ kJ��mol-1��
(4)��x��y��z�ֱ��ʾH2O��H2S��H2Se�ķе�(��)����x��y��z�Ĵ�С��ϵ��______________�����ж�������____________________________________________________________��
(1)2
(2)2H2O![]() H3O++OH-
H3O++OH-
(3)20
(4)x��z��y ˮ���Ӽ���������H2Se�ķ��Ӽ�����������H2S
������(1)ÿ��ˮ�������ڵ�4��ˮ��������������ӣ��γ�4���������ÿ�������2��ˮ�������У�����1 mol������2 mol�����
(2)ˮ�Ǽ����ĵ���ʣ��ɵ�������OH-��H3O+��
(3)���������������![]() =20 kJ��mol-1
=20 kJ��mol-1
(4)������ɺͽṹ���Ƶ����ʣ���Է�������Խ���Ӽ�������Խ�е�Խ�ߣ���ˮ���Ӽ仹������������Ӽ���������е���ߡ�


 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��(1)1 mol������ __________mol���������?
(2)ˮ���ӿɵ����������ֺ�����ͬ�����������ӣ�����뷽��ʽΪ��_______________________��

ͼ2��5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

���Ľṹͼ
(1)1 mol������__________mol���������
(2)ˮ���ӿɵ����������ֺ�����ͬ�����������ӣ�����뷽��ʽΪ��_______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
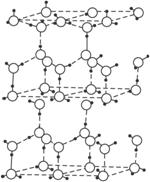
ͼ2-13
��1����1 mol������___________mol���������
��2��ˮ�����пɵ����������ֺ�����ͬ����������������뷽��ʽΪ___________��
��3���ڱ��Ľṹ�У�ÿ��ˮ���������ڵ�4��������������ӡ��ڱ��г�����⣬�����ڷ��»�����11 kJ��mol-1������֪������������51 kJ��mol-1������������������__________kJ��mol-1��
��4����x��y��z�ֱ��ʾH2O��H2S��H2Se�ķе㣨�棩����x��y��z�Ĵ�С��ϵ______________________�����ж�������______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ˮ���Ӽ����һ�ֽС�����������ã����ڷ����߶�˹���뻯ѧ��֮�䣩�˴˽�϶��γ�(H2O)n���ڱ���ÿ��ˮ���ӱ�4��ˮ���Ӱ�Χ�γɱ��ε��������壬ͨ�����������ӳ��Ӵ�ķ��Ӿ���һ�Σ���ṹʾ��ͼ��ͼ��ʾ
ˮ���Ӽ����һ�ֽС�����������ã����ڷ����߶�˹���뻯ѧ��֮�䣩�˴˽�϶��γ�(H2O)n���ڱ���ÿ��ˮ���ӱ�4��ˮ���Ӱ�Χ�γɱ��ε��������壬ͨ�����������ӳ��Ӵ�ķ��Ӿ���һ�Σ���ṹʾ��ͼ��ͼ��ʾ
��1mol������ mol�������
��ˮ���ӿɵ����������ֺ�����ͬ����������������뷽��ʽΪ�� ��
���ڱ��Ľṹ�У�ÿ��ˮ���������ڵ�4��ˮ��������������ӡ��ڱ������г�����⣬�����ڷ����߶�˹����11kJ•mol�D1������֪������������51 kJ•mol�D1���������������������� kJ•mol�D1
����x��y��z�ֱ��ʾH2O��H2S��H2Se�ķе㣨�棩����x��y��z�Ĵ�С��ϵ�� �����ж�������__________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com