
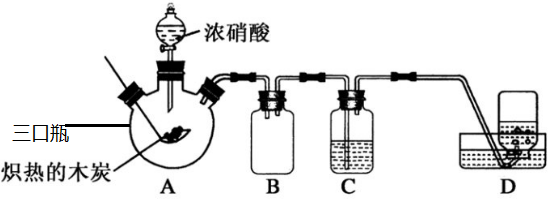
���� ��1��̼��Ũ�����ڼ��ȵ������·�Ӧ���ɶ�����̼�ͺ���ɫ�Ķ���������
��2��װ��C��ʢ������Ba��OH��2��Һ��������̼ͨ�����У�����̼�ᱵ�����������������������Һͬ��Ҳ�������ն�����̼����̼��Ƴ�����
��3������װ��ͼ��֪��װ��B������ȫƿ�����Է�ֹҺ�嵹����
��4����A���������ƿ��������NO����Ȼͨ�����ڹ۲���Կ�������ɫ���������������ж�������Ⱦ������
B��ʪ�����ɫʯ���Բ�������һ��������������
C���������ǵ�ľ�����뼯��ƿ�У��۲�ľ���Ƿ�ȼ�������жϼ���ƿ���Ƿ���������
�������ڼ��ȵ������·ֽ��������������ˮ��������
��� �⣺��1��̼��Ũ�����ڼ��ȵ������·�Ӧ���ɶ�����̼�ͺ���ɫ�Ķ������������Կɹ۲쵽����ƿ���������ɫΪ����ɫ����Ӧ����ʽΪC+4HNO3��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+4NO2��+2H2O��
�ʴ�Ϊ������ɫ��C+4HNO3��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+4NO2��+2H2O��
��2��װ��C��ʢ������Ba��OH��2��Һ��������̼ͨ�����У�������ɫBaCO3�����������������������Һͬ��Ҳ�������ն�����̼����̼��Ƴ�������������Ca��OH��2��Һ����Ba��OH��2��Һ��
�ʴ�Ϊ��BaCO3���ܣ�Ŀ�Ķ���Ϊ�˳���CO2��
��3������װ��ͼ��֪��װ��B������Ϊ������ȫƿ�����壬��ֹ��Һ������
�ʴ�Ϊ�����壬��ֹ��Һ������
��4����A���������ƿ��������NO����Ȼͨ�����ڹ۲���Կ�������ɫ���������������ж�������Ⱦ�������ʲ����ʣ�
B��ʪ�����ɫʯ���Բ�������һ���������������ʲ����ʣ�
C���������ǵ�ľ�����뼯��ƿ�У��۲�ľ���Ƿ�ȼ�������жϼ���ƿ���Ƿ����������ʺ��ʣ�
��ѡC��
�������ڼ��ȵ������·ֽ��������������ˮ��������������������Դ��Ũ����������ʱ�ֽ��������������������ˮ֮�����ɵ�HNO3�ֽ���������
�ʴ�Ϊ��Ũ����������ʱ�ֽ��������������������ˮ֮�����ɵ�HNO3�ֽ���������
���� ���⿼������ʵ�鷽�������ۼ�ʵ��װ�õ��ۺ�Ӧ�ã�Ϊ��Ƶ���㣬����װ�õ����á�������Ʊ�ʵ���Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע���Ԫ�ػ�����֪ʶ���ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ�


 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
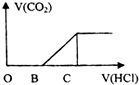 ��ijNaOH��Һ��ͨ��CO2����� �õ���ҺM����CO2ͨ�������ͬ����ҺM�����Ҳ��ͬ������M����μ������ᣬ�������������V��CO2���������������V��HCl���Ĺ�ϵ��ͼ��ʾ�������з������ж���ȷ���ǣ�����CO2�ܽ⣩��������
��ijNaOH��Һ��ͨ��CO2����� �õ���ҺM����CO2ͨ�������ͬ����ҺM�����Ҳ��ͬ������M����μ������ᣬ�������������V��CO2���������������V��HCl���Ĺ�ϵ��ͼ��ʾ�������з������ж���ȷ���ǣ�����CO2�ܽ⣩��������| A�� | ��OB=0������ҺMΪNa2CO3��Һ | |
| B�� | ��OB=BC�����γ���ҺM��������Ӧ�����ӷ���ʽΪOH-+CO2�THCO3- | |
| C�� | ��3OB=BC������ҺM��c��NaHCO3��=2c��Na2CO3�� | |
| D�� | ��OB��BC������ҺM�д������ڵ�������ΪCO32-��HCO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 14C�� 12C����ͬλ�أ�O2��O3����Ԫ�ص�ͬ�������� | |
| B�� | ����������Ҫ����������ʴ����п��Ƥ�Ʋ���������ʧȥ�������� | |
| C�� | ʯ�͵ķ���ú�ĸ��������Һ��������������仯 | |
| D�� | SO2��NO2�������������Na2O��Fe3O4���ڼ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼ�����ʽ�ζ��ܺ��ձ��зֱ�ע��0.2mol•L-1Ba��OH��2��Һ��0.1mol•L-1ϡ�����50mL������ϡ�����еμӼ���ʯ����Һ������ͼװ�����Ӻã�
��ͼ�����ʽ�ζ��ܺ��ձ��зֱ�ע��0.2mol•L-1Ba��OH��2��Һ��0.1mol•L-1ϡ�����50mL������ϡ�����еμӼ���ʯ����Һ������ͼװ�����Ӻã��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| Ԫ�ش��� | A | B | C | D | E | F | G | H | I | J | K |
| �������ϼ� | -1 | -2 | +5��-3 | +4��-4 | +6��+4��-2 | +4��-4 | +5��-3 | +3 | +2 | +1 | +1 |
| ԭ�Ӱ뾶/pm | 71 | 74 | 75 | 77 | 102 | 117 | 110 | 143 | 160 | 186 | 152 |
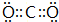 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C14H18O5 | B�� | C14H16O4 | C�� | C14H22O5 | D�� | C14H10O5 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com