
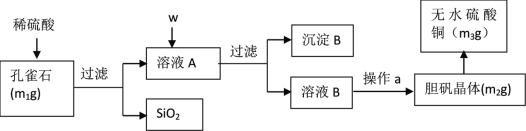
����Ŀ����ȸʯ����Ҫ�ɷ�ΪCu2��OH��2CO3��������FeO��Fe2O3��SiO2����ijС��ͬѧ��ʵ�����Կ�ȸʯΪԭ���Ʊ����������ⶨ���õ����нᾧˮ�ĺ�����ʵ�鲽�����£�
���ṩ�Լ���NaOH��Һ�� H2O2��Һ�� CuO ��Na2CO3��
���������գ�
��1����ȸʯ����Ҫ�ɷ���ϡ���ᷴӦ�����ӷ���ʽΪ________��
��2�����Լ�����˳��w���������Լ�����Ϊ__________������B�ijɷ�Ϊ_______��
��3��a��������Ϊ������������_______�����ˡ�ϴ�ӡ�__________�����ձ���©��֮����˻���Ҫ��һ�����������������ڴ˲����е���Ҫ������_________��
��4����������ҺA��Fe2+���Լ���___________��ѡ����ţ�������ⶨFe2+�ĺ�������Ҫ������ƿ����ij����Һ������������С����ݡ��IJ�����_____��
a NaOH��aq�� b Na2CO3��aq�� c ����KI��aq�� d ����KMnO4��aq��
��5���ڲⶨ���õ�����CuSO4��xH2O���нᾧˮxֵ��ʵ������У����ⶨ�����ʵ�����Ϊ1.5%�����ܵ�ԭ����_______________��
a �����¶ȹ��� b ��������Ŀ����ϴ�
c ���Ⱥ���ڿ�������ȴ d ���ȵ�������ʱ�о���������н���
��6����С��һλͬѧ����ʵ���������Ʒ�п�ȸʯ����������Ϊ�� ����һλͬѧ��ͬ�⣬��ԭ����___________��
����һλͬѧ��ͬ�⣬��ԭ����___________��
���𰸡�Cu2��OH��2CO3+4H+��2Cu2++3H2O+CO2�� H2O2��Һ�� CuO Fe��OH��3�� CuO ��ȴ�ᾧ ��Ȼ���� ���� d ��ˮ����̶���2��3cm�����ý�ͷ�ιܵμ�����ˮ��ֱ����Һ����������̶�����ƽ a��d �ڳ��ӹ����м�����CuO����ʹ���ƫ��
��������
m1g��ȸʯ����Ҫ�ɷ�ΪCu2��OH��2CO3��������FeO��Fe2O3��SiO2��������ϡ������˵õ����������������ҺAΪ����ͭ������������������������WΪ��������������������Ϊ�����ӣ���������ͭ������Һ��pH���������ӣ����˵õ�����BΪ������������������ͭ����ҺBΪ����ͭ��Һ��ͨ������Ũ������ȴ�ᾧ������ϴ�ӡ�����õ�����ͭ����m2g������ʧȥ�ᾧˮ�õ�����ͭ����m3g���ݴ˽��
��1����ʽ̼��ͭ�����ᷴӦ��������ͭ��ˮ�Ͷ�����̼����Ӧ�����ӷ���ʽΪ��Cu2��OH��2CO3+4H+=2Cu2++3H2O+CO2�����ʴ�Ϊ��Cu2��OH��2CO3+4H+=2Cu2++3H2O+CO2����
��2�����Լ�����˳��w���������Լ�����Ϊ�����������������������������ӣ�������������־���ӣ���������ͭ������ҺpHʹ������ȫ�����������˵õ�����BΪFe��OH��3��CuO���ʴ�Ϊ��H2O2��Һ��CuO��Fe��OH��3��CuO��
��3������ҺB�л������ͭ���壬ֱ�Ӽ������ɻᵼ������ͭʧȥ�ᾧˮ��Ӧ�ò��õIJ�������Ϊ������Ũ������ȴ�ᾧ�����ˡ���Ȼ������ձ���©��֮����˻���Ҫ��һ��������Ϊ���������������ڴ˲����е���Ҫ�������������ʴ�Ϊ����ȴ�ᾧ����Ȼ���������
��4����ҺA�к��������Ӻ������ӣ�a��NaOH��Һ����Fe2+��Һ�м���NaOH��Һ��Fe2+��OH-��Ӧ����Fe��OH��2��Fe2++2OH-=Fe��OH��2����Fe��OH��2���ȶ��ױ���������ΪFe��OH��3��4Fe��OH��2+O2+2H2O=4Fe��OH��3����ɫ��Fe��OH��2������ɺ��ɫFe��OH��3����Fe2+��Һ�м���NaOH��Һ�۲쵽���ɵİ�ɫ����Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ��Fe3+��OH-��ӦFe3++3OH-=Fe��OH��3�������ɺ��ɫFe��OH��3�����߷�Ӧ������ţ����ܼ������Һ�д���Fe2+����a����
b��Na2CO3��aq���������������ɳ�������������˫ˮ�������������������Ͷ�����̼����Һ���Dz��ܼ����������ӣ���b����
c������KI��aq���������ӷ���������ԭ��Ӧ���ɵⵥ�ʺ��������ӣ���Һ��ɫ������ܼ����������ӣ���c����
d������KMnO4��aq������ΪFe2+���н�ǿ�Ļ�ԭ����ʹ����KMnO4��Һ��ɫ��Fe3+���ܣ����ܹ�������KMnO4��Һ����Fe2+��Fe3+����d��ȷ��
����ⶨFe2+�ĺ�������Ҫ������ƿ����ij����Һ����ˮ����̶���2��3cm�����ý�ͷ�ιܵμ�����ˮ��ֱ����Һ����������̶�����ƽ���ʴ�Ϊ��d����ˮ����̶���2��3cm�����ý�ͷ�ιܵμ�����ˮ��ֱ����Һ����������̶�����ƽ��
��5���ⶨ���õ�����CuSO4xH2O���нᾧˮxֵ��Ӧ���������������������;�������������Ⱥ����������������Ⱥ��ٳ���һ���������������ж������Ƿ������������Χ�ڼ�����ֵ�Ƿ�������0.1g���������ٳ���4�Σ�
a�������¶ȹ��ߣ��ᵼ������ͭ�ֽ⣬�����仯�ϴ��½��ƫ��a��ȷ��
b����������Ŀ����ϴᵼ�¾�����ȷֽⲻ��ȫ�������仯ƫС�����ƫС��b����
c�����Ⱥ���ڿ�������ȴ�������տ����е�ˮ�����γɾ��壬���²ⶨ���ƫС��c����
d�����ȵ�������ʱ�о���������н��������²ⶨ�Ľᾧˮ������ƫ�ⶨ��xֵƫ��d��ȷ��
�ʴ�Ϊ��ad��
��6������Һ�м���CuO������Һ��pH��������ΪFe��OH��3�����Ե����е�ͭԪ�ز��Ƕ�������Ʒ����˸�С��һλͬѧ����ʵ���������Ʒ��CuO����������ƫ�ʴ�Ϊ���ڳ��ӹ����м�����CuO����ʹ���ƫ��


 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д� �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ���׳ص��ܷ�ӦʽΪ��N2H4+O2=N2+H2O�����й��ڸõ�ع���ʱ��˵����ȷ������ ��

A. ��װ�ù���ʱ��Ag�缫������������
B. �׳��и�����ӦΪN2H4-4e-=N2+4H+
C. �׳غ��ҳ��е���Һ��pH����С
D. ���׳�������0.1molN2H4ʱ���ҳ���������������6.4g����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
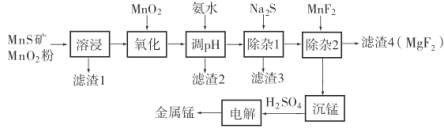
����Ŀ���̲����ڹ�ҵ����;�㷺�����������ڱ������Ԫ�أ������彡��������Ҫ�����á�һ������Ȼ�������̷������̿���Fe��Al��Mg��Zn��Ni��Si��Ԫ�أ�Ϊԭ���Ʊ������̵�������ͼ��ʾ����ش��������⣺
��ؽ�������![]() �γ��������������pH��Χ���£�
�γ��������������pH��Χ���£�
�������� |
|
|
|
|
|
|
|
��ʼ����pH | 8.1 | 6.3 | 1.5 | 3.4 | 8.9 | 6.2 | 6.9 |
������ȫ��pH | 10.1 | 8.3 | 2.8 | 4.7 | 10.9 | 8.2 | 8.9 |
(1)���ܽ������������Լ���________���ѧʽ����
(2)����pH��������������Һ��pH��ΧӦ����Ϊ________~6֮�䡣
(3)������2������Ҫ�ɷ���________________________________���ѧʽ����
(4)![]() ���������̡��Լ��������ʵ�ˮ��Һ�ʼ��ԣ�����Һ������Ũ���ɴ�С��˳��Ϊ________________________________��
���������̡��Լ��������ʵ�ˮ��Һ�ʼ��ԣ�����Һ������Ũ���ɴ�С��˳��Ϊ________________________________��
(5)�����̡��õ�![]() ������д���÷�Ӧ�����ӷ���ʽ________________��
������д���÷�Ӧ�����ӷ���ʽ________________��
(6)�����������Һ�Ʊ�������ʱ�������ĵ缫��ӦΪ________������ĵ������Һ�ɷ���________�������ʹ�á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ҷ��ᣬ�ֳƲ��ᣬͨ���ڿ������ױ����������ʡ��������ӽᾧˮ��H2C2O4��2H2O��ȴ���ڿ������ȶ����ڡ��ڷ�����ѧ�г���H2C2O4��2H2O��KMnO4�ĵζ��������й���H2C2O4��˵����ȷ����
A�������Ƕ�Ԫ���ᣬ����뷽��ʽΪH2C2O4![]() 2H++C2O
2H++C2O![]()
B������ζ�KMnO4�����к͵ζ�������ʯ����ָʾ��
C���Ҷ����ͨ����ϩ�����ӳɡ�ˮ�⡢�����������Ƶ�
D����ŨH2SO4�μӵ��Ҷ�����ʹ֮��ˮ�ֽ⣬�ֽ������CO2��H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�Ͻ�Cl2ͨ��KOH����Һ����ȡKClO3������KClO����ʵ����ģ�������Ʊ�����18mol KOH����Һ��ͨ��Cl2����ַ�Ӧ������Һ��n��Cl-��=12mol��������Һ�������ɣ����õ�������KClO3�����ʵ�������Ϊ
A.1.5molB.2.1molC.2.4 molD.3.0 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ɫֲ��Ĺ�����ã��ǵ�����������ձ�Ļ�ѧ�仯��Ҳ�ǽ�___________��ת��Ϊ________ �ܵ�����Ҫ;����ֲ��ͨ��������ã���������̼��ˮת��Ϊ�����ǣ�C6H12O6�������ų���������д���÷�Ӧ�Ļ�ѧ����ʽ��________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���N�Ľṹ�к���������Ԫ������ϳ�·�����¡�

��֪��RCH=CH2+CH2=CHR��![]() CH2=CH2+RCH=CHR��
CH2=CH2+RCH=CHR��
��ش��������⣺
��1��F�����к��������ŵ�����Ϊ_______��B�Ľṹ��ʽΪ____________��
��2��G��H�Ļ�ѧ����ʽ______________���䷴Ӧ����Ϊ_____��
��3��D��һ���������ܺϳɸ߷��ӻ�����÷�Ӧ�Ļ�ѧ����ʽ____________��
��4��A ��5000C��Cl2����������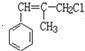 ��������
��������![]() ��
�� ��ԭ����_________��
��ԭ����_________��
��5��E��ͬ���칹������ʹFeCl3��Һ��ɫ����_______�֡�
��6��N�Ľṹ��ʽΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ظ����(K2Cr2O7)Ϊ��;����ĺ���������������Ʊ�������������ϣ������������ơ��л��ϳɵȡ����������Ҫ�ɷֿɱ�ʾΪFeO��Cr2O3��������SiO2��Al2O3�����ʣ��Ը�����Ϊԭ���Ʊ�K2Cr2O2����Ĺ�����ͼ��ʾ
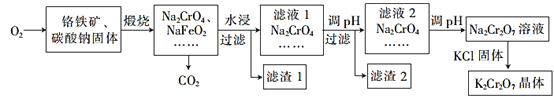
��������ش��������⣺
(1)����ʱ�������������ӷ�Ӧ����Na2CrO4�� NaFeO2��ͬʱ����SiO2��Al2O3��Na2CO3������Ӧ������Al2O3������Ӧ�Ļ�ѧ����ʽΪ___��
(2) NaFeO2��ˮǿ��ˮ����������1����Ӧ�����ӷ���ʽΪ______��
(3)��Һ1����Һ2���������ҺpH����Һ1����pHΪ7~8��Ŀ��Ϊ_____����Һ2����pHԼΪ5��Ŀ����_____��
���� | �ܽ��(g/100g) | ||
0�� | 40�� | 80�� | |
KCl | 28 | 40.1 | 51.3 |
NaCl | 35.7 | 36.4 | 38 |
K2Cr2O7 | 4.7 | 26.3 | 73 |
Na2Cr2O7 | 163 | 215 | 376 |
(4)��Na2Cr2O7��Һ�м���KCl���壬����K2Cr2O7���塣�����˷��ܹ��Ƶ�K2Cr2O7�����ԭ��____��Ϊ�˴���Һ�еõ��϶�K2Cr2O7����IJ���������____��____�����ˡ�ϴ�ӡ����
(5)KCr2O7��Ʒ�����ⶨ��ȷ��ȡ����2.5g�����250ml��Һ������Һ����ȡ25.00mL��Һ�ڵ���ƿ�У�����10mL2mol��L-1���ᡢ2gKI�����ڰ���5min������l00mˮ����0.2000mol��L��1Na2S2O3��Һ�ζ�����Һ�ʻ���ɫ���ټ���3mL������Һ�����ζ�����ɫ��ȥ��������ɫ������ƽ��ʵ�����Σ�ƽ������NaS2O3��Һ�����Ϊ25.00mL.K2Cr2O7��Ʒ�Ĵ���Ϊ______����֪�йط�Ӧ���£�![]() ��
��![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ϡ��ˮ�д�������ƽ�⣺NH3+H2O ![]() NH3��H2O
NH3��H2O![]() NH4+ + OH����������ϡ��ˮ�м�����������ʱ�������������( )
NH4+ + OH����������ϡ��ˮ�м�����������ʱ�������������( )
A.�����Ȼ�粒��壬ƽ�������ƶ�����ҺpH����
B.ͨ����ఱ����ƽ�������ƶ�����Һ������ǿ
C.�����������ƹ��壬ƽ�������ƶ�����Һ��������ǿ
D.�������� NaCl��Һ��ƽ�ⲻ�ƶ�����ҺpH����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com