
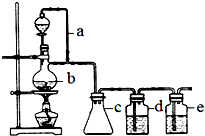
 ij�о���ѧϰС��Ϊ�ϳ�1-�������������ϵ�֪һ���ϳ�·�ߣ�
ij�о���ѧϰС��Ϊ�ϳ�1-�������������ϵ�֪һ���ϳ�·�ߣ����� ��1�������װ���У�a�����ñ��ַ�Һ©������ƿ�ڵ���ѹ��ȣ��Ա�֤��Һ©���ڵ�Һ����˳��������ƿ�У�
��2��ʵ��ʱ��װ��b�м��뼸����ʯ��ֹ�ٷУ����Ⱥ���δ�ӷ�ʯ��Ӧ��ȴ���ٲ��ӣ�
��3��c��Ҫ����ȫƿ�����ã��Է�ֹ������
��4�������ϩ������SO2��CO2��ˮ������ɵĻ��������ɷ�ʱ��Ӧ����ѡ����ˮCuSO4����ˮ������Ȼ���â�Ʒ����Һ����SO2�����âٱ���Na2SO3��Һ��ȥSO2��Ȼ���â�ʯ��ˮ����CO2���â�����KMnO4��Һ�����ϩ��Ũ�����2-�����ڼ���ʱ����������ԭ��Ӧ���ɶ�����������̼��ˮ��
��5������NaHSO3��Һ�γɳ�����Ȼ��ͨ�����˼��ɳ�ȥ��1-���������ѵķе����ܴ���˿���������������뿪��
��6����ÿһ���������ʻ���ʧ�ʶ�ת��Ϊ��ʼԭ�ϵ������ʻ���ʧ�ʣ��ݴ˼���ԭ�ϵ�ʵ�������ʣ�����Ԫ���غ����1-������������
��� �⣺��1��Ϊ��ʹ��Һ©���е�Һ����˳�������£��ͱ��豣�ַ�Һ©������ƿ�ڵ���ѹ��ȣ����a�������DZ��ֺ�ѹ��
�ʴ�Ϊ��ƽ��ѹǿ��ʹҺ��˳�����£�
��2��ʵ��ʱ��װ��b�м��뼸����ʯ��ֹ�ٷУ����Ⱥ���δ�ӷ�ʯ��Ӧ��ȴ���ٲ��ӣ�
�ʴ�Ϊ����ֹ���У�ֹͣ������ȴ���ټ����ʯ��
��3��c��Ҫ����ȫƿ�����ã��Է�ֹ������
�ʴ�Ϊ������������ȫƿ���ã�
��4�������ϩ����������KMnO4��Һ������SO2����������KMnO4��Һ��ɫ��Ʒ����Һ��ʯ��ˮ������CO2����ʯ��ˮ������ˮ����������ˮCuSO4�������ڼ���������������迼���Լ���ѡ���˳��ֻҪͨ����Һ���ͻ����ˮ����������ȼ���ˮ������Ȼ�����SO2���ڼ���֮���ȥSO2����SO2�����ñ���Na2SO3��Һ��������CO2�ͱ�ϩ�����˳��Ϊ�ݢ٢ۢڣ�Ũ�����2-�����ڼ���ʱ����������ԭ��Ӧ���ɶ�����������̼��ˮ����Ӧ�ķ���ʽΪCH3CHOHCH3+9H2SO4$\frac{\underline{\;\;��\;\;}}{\;}$9SO2+3CO2+13H2O��
�ʴ�Ϊ���ݢ٢ۣ�CH3CHOHCH3+9H2SO4$\frac{\underline{\;\;��\;\;}}{\;}$9SO2+3CO2+13H2O��
��5����Ʒ�к�������ȩ��������������Ϣ���ñ���NaHSO3��Һ�γɳ�����Ȼ��ͨ�����˼��ɳ�ȥ�����ڱ���NaHSO3��Һ�ǹ����ģ����Լ������ѵ�Ŀ������ȡ��Һ�е�1-��������Ϊ1-���������ѵķе����ܴ���˿���������������뿪��
�ʴ�Ϊ������NaHSO3��Һ�����ˣ���ȡ������
��6����ÿһ���������ʻ���ʧ�ʶ�ת��Ϊ��ʼԭ�ϵ������ʻ���ʧ�ʣ���ԭ�ϵ�ʵ��������Ϊ85%��80%��75%������Ԫ���غ㼰��Ӧ����ʽ��֪1-����������Ϊ85%��80%��75%��$\frac{60g}{60g/mol}��74g/mol$=37.74g��
�ʴ�Ϊ��37.74��
���� ���⿼���л���ϳɷ�������ƣ���Ŀ�ѶȽϴ��ۺ��Խ�ǿ������ʱע��������ʵķ��롢�ᴿ�������������ʵ����ʵ���ͬ�ǽ�����Ĺؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ϡHNO3�еμ�Na2SO3��ҺSO32-+2H+�TSO2��+H2O | |
| B�� | CuSO4��Һ��H2S��Ӧ�����ӷ���ʽ��Cu2++S2-�TCuS�� | |
| C�� | ��������ˮ��Cl2+H2�T2H++Cl-+ClO- | |
| D�� | ��CuSO4��Һ�м���Na2O2��2Na2O2+2Cu2++2H2�T4Na++2Cu��OH��2��+O2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ij��ѧ����С��ʹ����˿������������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��У�
ij��ѧ����С��ʹ����˿������������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��У�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2Al+6HCl�T2 AlCl3+3H2 | B�� | 4Na+O2�T2Na2O | ||
| C�� | MgO+H2SO4�TMgSO4+2H2O | D�� | Ba��OH��2+H2SO4�TBaSO4��+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ�� | ���� | ���� |
| A | ��ϡ���������������У���ַ�Ӧ��μ�KSCN��Һ | ���������ɣ���Һ�ʺ�ɫ | ϡ���ὫFe����ΪFe3+ |
| B | ��ͭ�ۼ���1.0mol/LFe2��SO4��3��Һ�� | ��Һ�������к�ɫ������� | ��������ͭ���� |
| C | ������ǯ��סһС����ɰֽ��ϸ��ĥ���������ھƾ����ϼ��� | �ۻ����Һ̬���������� | ���������۵�ϵ� |
| D | ����ͬ�����£��ֱ����Na2CO3�����NaHCO3���� | NaHCO3����ֽ⣬��������ʹ����ʯ��ˮ����ǣ�Na2CO3���岢û�зֽ� | Na2CO3������ȶ��Ա�NaHCO3�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������ˮ��Ӧ���������ͼ� | |
| B�� | ���ܴ�����ͭ��Һ���û���ͭ | |
| C�� | ȥ���������ͭ�Ʋ����ѡ��ϡ���� | |
| D�� | ��˿�����ڿ����л����ڴ����ж����Է���������ԭ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��FeCl3��Һ��ʴͭ��·�壺Cu+Fe3+�TCu2++Fe2+ | |
| B�� | Na2O2��H2O��Ӧ�Ʊ�O2��2Na2O2+2H2O�T4Na++4OH-+O2�� | |
| C�� | ����������ˮ�Ʊ������Cl2+H2O�T2H++Cl-+ClO- | |
| D�� | ��Ũ�����ữ��KMnO4��Һ��H2O2��Ӧ��֤��H2O2���л�ԭ�ԣ�2MnO4-+6H++5H2O2�T2Mn2++5O2��+8H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com