

| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
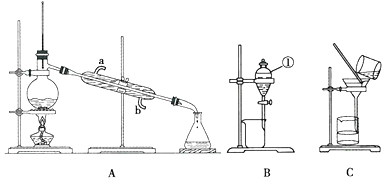
���� a����1������NaOH����Һ�ζ����յ㣬�ζ���ʢ�ŵ�������������Һ��ѡ��ʽ�ζ��ܣ�
�ڵζ�����δ�ñ�Һ��ϴ��ֱ�Ӽ����Һ��ϡ����ҺŨ�ȼ�С����ƿ��ˮ�Եζ������Ӱ�죻
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲���ƿ����Һ����ɫ�仯�����ж��ζ��յ㣻
������Һ��ɫ�����仯���Ұ�����ڲ���ɫΪ�ζ��յ㣻
��2�����ݵζ�ǰ����������������Һ�������ƽ��ֵ�����4NH4++6HCHO�T4H++��CH2��6N4+6H2O��H++OH-=H2O���㣻
b��A�����β����������½���Һ�壬�Ҳ����ȣ�
B���¶ȼƲ��ܽӴ��ձ��ײ���
C���к���Ӧ��ǿ���ǿ���ϡ��Һ��
D���к��Ȳⶨ��ʵ����Ҫ��Ͳ���¶ȼơ����β����������
��� �⣺a����1������NaOH����Һ�ζ����յ㣬�ζ���ʢ�ŵ�������������Һ��ѡ��ʽ�ζ��ܣ���ѡ�ң�
�ʴ�Ϊ���ң�
�ڵζ�����δ�ñ�Һ��ϴ��ֱ�Ӽ����Һ��ϡ����ҺŨ�ȼ�С�����ı�Һ����������������ʵ�������4NH4++6HCHO�T4H++��CH2��6N4+6H2O����Ӧ��֪�ⶨ��Ԫ�غ���ƫ�ߣ���ƿ��ˮ�Եζ������Ӱ�죬
�ʴ�Ϊ��ƫ�ߣ���Ӱ�죻
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲���ƿ����Һ����ɫ�仯���ʴ�Ϊ��B��
���������Ƶζ�����Һ���ﵽ�յ�ʱ����̪��ɫ�仯Ϊ��ɫ�仯Ϊ��ɫ��������ڲ���ɫ���ʴ�Ϊ���ޣ��죻
��2����Ʒ1.5000g����ӦΪ4NH4++6HCHO�T4H++��CH2��6N4+6H2O�����������ӵ����ʵ����͵�Ԫ�����ʵ�����ͬ������ͼ�����ݷ�����֪������ʵ������������������Һ����ֱ�Ϊ��20.01mL��19.99mL��20.00ml�����ξ���Ч������������Һ��ƽ�����Ϊ20.00mL��������кͷ�Ӧ��֪�����������ʵ���=�����������ʵ���=0.1000mol•L-1��20.00ml��10-3L/ml=2.00��10-3mol��
250mL��Һ�е�Ԫ�����ʵ��������������ʵ�����ͬ������Ʒ�е�����������Ϊ$\frac{2��1{0}^{-3}mol��14g/mol��\frac{250}{25}}{1.5000g}$��100%=18.85%��
�ʴ�Ϊ��18.85%��
b��A�����β����������ܼӿ췴Ӧ���ʣ���Сʵ������A��ȷ��
B���¶ȼ�ˮ������ձ��ڵ���ˮ���¶ȣ����ܽӴ��ձ��ײ��Ӵ��ձ��ײ�����B����
C�����������ᣬ�������ȣ�������к�����ֵƫС����C����
D���к��Ȳⶨ�ò�����ƽ����D����
��ѡA��
���� ���⿼�����ʺ����IJⶨ/�к��ȵIJⶨ��Ϊ��Ƶ���㣬�������ʵ����ʡ��ζ�������������ʹ�á���Ϸ�Ӧ�ļ���Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע�����ݴ����������ļ��㣬��Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1-��ϩ�ļ���ʽ�� | B�� | ��ȩ�����ģ�ͣ� | ||
| C�� | �ǻ��ĵ���ʽ��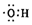 | D�� | �����ǵĽṹ��ʽ��C6H12O6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������ˮ��Ӧ��������ת��4NA�������������Ϊ32g | |
| B�� | ��״���£�22.4L�����к���NA������ | |
| C�� | 4g��ϩ���ϩ�Ļ�����庬��0.15NA��̼ԭ�� | |
| D�� | 50mL18.4mol•L-1Ũ����������ͭ�ȷ�Ӧ������SO2������ĿΪ0.46NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 25��ʱ����������Ũ�ȵ�NaOH��Һ����Һ��pHһ������7 | |
| B�� | c��C2O42- ����c�� OH-�� | |
| C�� | c��Na+��+c��H+����c��C2O42- ��+c�� HC2O4-��+c�� OH-�� | |
| D�� | c��Na+����c�� HC2O4-����c��H2C2O4����c��C2O42- �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1molCl2�μӷ�Ӧ����һ���õ�2NA������ | |
| B�� | ��״���£���22.4LNO��11.2LO2��Ϻ��Եõ�NA��NO2���� | |
| C�� | ���ڳ�ѹ�£�1L0.1mol/LHF��Һ�к���0.1NA��H+ | |
| D�� | 25�棬1.7g�ǻ�����������ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ԭ�ӵ�L���Ӳ�����2������ | B�� | ���������� | ||
| C�� | ��Ľ����Ա����Ľ�����ǿ | D�� | ���ԭ�Ӱ뾶��̼��ԭ�Ӱ뾶С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

 ��
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com