
�����ʵ�����Ϊ0.1 mol��CuCl2��H2SO4����ˮ�Ƴ�100 mL�Ļ����Һ����ʯī���缫��⣬���ռ����缫�����������壬һ��ʱ����������ռ�������������ͬ�����������ͬ��������������ȷ���� (����)
A����·�й�ת��0.6NA������
B�������õ���������O2�����ʵ���Ϊ0.2 mol
C��������������3.2 g
D������ʣ����Һ�������Ũ��Ϊ1 mol��L��1

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����к���Ԫ�ء��Ӻ�������ȡ�������²���:��ͨ������Cl2���ڽ��������ճɻҺ��ˮ���裻�ۼ�CCl4�����÷�Һ©����Һ���ݹ��ˡ������IJ���˳����
A.�٢ڢۢܢݡ� B.�ڢݢ٢ۢܡ� C.�٢ۢݢڢܡ� D.�ڢ٢ۢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������Ե缫����500 mL AgNO3��Һ�У�ͨ���⡣�����Һ��pH��6.0��Ϊ3.0ʱ(�������������û��H2�ų����ҵ��Һ�ڵ��ǰ������仯���Ժ��Բ���)���缫�����������������Ϊ (����)
A��27 mg B��54 mg
C��106 mg D��216 mg
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ը����Ϊԭ�ϣ��绯ѧ���Ʊ��ظ���ص�ʵ��װ��ʾ��ͼ���£�
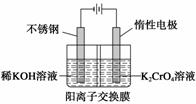
����˵������ȷ���� (����)
A���������ң������ĵ缫��ӦΪ2H2O��2e��===2OH����H2��
B���������ң�ͨ�����Һ���ɻ�ɫ��Ϊ��ɫ������Ϊ������H��Ũ������ʹƽ��2CrO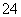 ��2H��Cr2O
��2H��Cr2O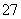 ��H2O�����ƶ�
��H2O�����ƶ�
C�����Ʊ��������ܷ�Ӧ�Ļ�ѧ����ʽΪ4K2CrO4��4H2O 2K2Cr2O7��4KOH��2H2����O2��
2K2Cr2O7��4KOH��2H2����O2��
D���ⶨ����Һ��K��Cr�ĺ�������K��Cr�����ʵ���֮��(nK/nCr)Ϊd�����ʱ����ص�ת����Ϊ1��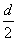
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ϩȩ�ṹ��ʽΪCH2=CH��CHO�����й������������в���ȷ���� ( )
A.�ܷ���������Ӧ������������ B.��ʹ��ˮ������KMnO4��Һ��ɫ
C.��һ���������ܱ��������� D.��һ����������H2��Ӧ����1�D����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ����(����)
A�������ǻ����л����Ϊ��
B���ܷ���������Ӧ���л��ﶼ��ȩ
C�������׳�ʯ̿�ᣬ���Ա�̼��ǿ
D�������ڼ��������µ�ˮ��̶ȴ������������µ�ˮ��̶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й����ʵ�������Ӧ�ò����Ӧ����
A��Na2O2�ֱܷ���H2O��CO2��Ӧ����������������������
B���轺��ס���ˮ����ǿ����������װʳƷ�ĸ����
C��K2FeO4����ǿ��ԭ���ұ���������Fe3����������ˮ�������;�ˮ
D��Һ������ʱ�����մ������ȣ�ʹ��Χ�¶ȼ��罵�ͣ���˿����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ɫ����Һ���������Ӧ�ų�H2�����ж��������ӣ�Mg2����Cu2����Ba2����H����Ag����SO ��HCO
��HCO ��OH����NO
��OH����NO ��Щ�ܴ������档
��Щ�ܴ������档
(1)������Al3��ʱ�����ܴ���____________________��
(2)������[Al(OH)4]��ʱ�����ܴ���____________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com