
| A�� | ˮ���������c��H+������=�ۣ���=�� | |
| B�� | ���ڡ�����Һ��Ϻ�pH=7��������Һ��������ڣ��� | |
| C�� | ������Ģ١��ڡ�����Һ�ֱ����������۷�Ӧ������H2����������� | |
| D�� | ����Һ�м���100mLˮ����Һ��pH���ۣ��ܣ��٣��� |
���� A���¶Ȳ��䣬ˮ�����ӻ��������䣬������Һ��PHֵ����ˮ�����������Ũ�ȣ�
B��pH=3��HCl��Һ��pH=11�İ�ˮ�У���ˮŨ�ȴ������
C������Ϊ���ᣬ������ڵ���ƽ�⣬�淴Ӧ���е�����������Ӽ���������Ӧ��
D����ˮϡ�ͣ�ǿ��ǿ����Һ��pH�仯�������������ڵ���ƽ��ϡ�ʹٽ����룮
��� �⣺A���١��ڵ�������Ũ����ͬ���ۡ��ܵ����������ӵ�Ũ����ͬ��������Һ������Ũ����ͬ����ͬ�����£�ˮ�����ӻ������Ƕ�ֵ�������ỹ�Ǽ����ˮ�ĵ��룬������������Һ����ˮ�����c��H+������=��=��=�ܣ���A����
B����ˮ������ֻ�в��ֵ��룬����c��NH3•H2O����c��OH-�����Ȼ�����ǿ����ʣ���������Һ��c��HCl��=c��H+����c��NH3•H2O����c��HCl����������ˮ�������Ϻ���Һ�����ԣ���������Һ��������ڣ��ۣ���B��ȷ��
C�����������ᣬ�Ȼ��������������ǿ����ʣ��١��ڡ���������Һ�����ʵ���Ũ�ȹ�ϵΪ���٣���=�ܣ����Ե�����Ģ١��ڡ�����Һ�ֱ������۷�Ӧ��������H2�������C����
D�����������ᣬ��ˮϡ�ͺ��ܴٽ�����ĵ��룬���Ԣ١���ϡ�ͺ���Һ��pHֵ7���ڣ��٣���ˮ�������ˮϡ�ͺ��ܴٽ���ˮ�ĵ��룬���Ԣۡ��ܡ�ϡ�ͺ���Һ��pHֵ�ۣ��ܣ�7��������������������Һ�зֱ����100mLˮ����Һ��pH���ۣ��ܣ��ڣ��٣���D����
��ѡB��
���� ���⿼����������ʵĵ��롢��Һϡ�ͺ�pHֵ��Դ�С�ıȽϵ�֪ʶ����Ŀ�Ѷ��еȣ����������ѧ���ķ��������Ŀ��飬Ϊ��Ƶ���㣬�״�ѡ����A��ע��������ˮ��Һ���Ǽ���Һ������ˮ�ĵ��룬�����ˮ���ܴٽ�ˮ�ĵ��룮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
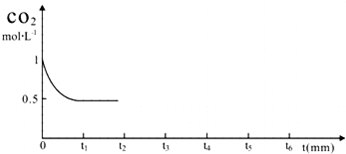
| ��Ӧʱ�� | CO2��mol�� | H2��mol�� | CH3OH��mol�� | H2O��mol�� | |
| ��ӦI ���º��� | 0min | 2 | 6 | 0 | 0 |
| 10min | 4.5 | ||||
| 20min | 1 | ||||
| 30min | 1 | ||||
| ��ӦII ���Ⱥ��� | 0min | 0 | 0 | 2 | 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
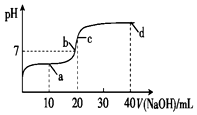 ����ʱ����20mL 0.1mol•L-1һԪ��HA��Һ�е���0.1mol•L-1��NaOH��Һ����Һ��pH�仯������ͼ��ʾ������˵����ȷ���ǣ�������
����ʱ����20mL 0.1mol•L-1һԪ��HA��Һ�е���0.1mol•L-1��NaOH��Һ����Һ��pH�仯������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | a�㣺c��HA����c��A-�� | B�� | b�㣺c��Na+��=c��A-��+c��HA�� | ||
| C�� | c�㣺c��H+��+c��HA��=c��OH-�� | D�� | d�㣺c��Na+����c��A-����c��OH-����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
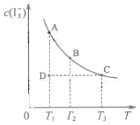 I2��KI��Һ�д�������ƽ�⣺I2��aq��+I-��aq��?I3-��aq��ijI2��KI�����Һ�У�I3-�����ʵ���Ũ��c��I3-�����¶�T�Ĺ�ϵ��ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬������I2 �Ļӷ���������˵����ȷ���ǣ�������
I2��KI��Һ�д�������ƽ�⣺I2��aq��+I-��aq��?I3-��aq��ijI2��KI�����Һ�У�I3-�����ʵ���Ũ��c��I3-�����¶�T�Ĺ�ϵ��ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬������I2 �Ļӷ���������˵����ȷ���ǣ�������| A�� | ��Ӧ I2��aq��+I-��aq��?I3-��aq����H��0 | |
| B�� | ״̬A��״̬B��ȣ�״̬A��c��I2���� | |
| C�� | ����Ӧ���е�״̬Dʱ��һ����v����v�� | |
| D�� | ���¶�ΪT1��T2����Ӧ��ƽ�ⳣ��K1��K2�� K1��K2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
 �������յ������Ϣ���������и��⣺
�������յ������Ϣ���������и��⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
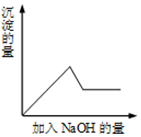 ��һ��ɫ��Һ�����п��ܺ���Fe3+��Cu2+��K+��Al3+��Mg2+��SO42-��Cl-�������еļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ����������ʵ�飬��������й��������£�
��һ��ɫ��Һ�����п��ܺ���Fe3+��Cu2+��K+��Al3+��Mg2+��SO42-��Cl-�������еļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ����������ʵ�飬��������й��������£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C��N��P | B�� | N��P��O | C�� | N��O��S | D�� | C��Si��P |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com