
����Ŀ����֪��̬��A�ڱ�״���µ��ܶ���1.16 g��L1��B�IJ���������������һ������ʯ�ͻ�����չˮƽ��G��һ�ָ߷��ӻ�������� A��B��C��D��E��F��G �������¹�ϵ��
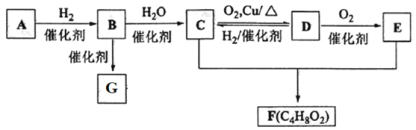
��ش�
(1)D�еĹ�����������_______________�� B�ĽṹʽΪ_________��
(2)д�� C+E��F��Ӧ�Ļ�ѧ����ʽ__________��
(3)д�� C��D��Ӧ�Ļ�ѧ����ʽΪ__________��
(4)��֪������ A ��һ�������¿ɺϳɲ���ʹ���� KMnO4 ��Һ��ɫ���л��д���úϳɷ�Ӧ�Ļ�ѧ����ʽ___________����Ӧ����Ϊ___________��
(5)�����й�������ȷ����_____________��
a. A��B��C��D��E��F��G��Ϊ�ǵ����
b. A������ԭ�Ӳ����ܴ���ͬһƽ����
c. ����ʱ��D ��������������ͭ����Һ��Ӧ����ש��ɫ����
d.75%(�������)�� C ˮ��Һ������ҽ������
e. ���̶���С���ƿ�Ͷ�� C �У��ƿ鸡��Һ���ϣ����д������ݲ���
���𰸡�ȩ�� ![]() CH3CH2OH+ CH3COOH
CH3CH2OH+ CH3COOH![]() CH3COOCH2CH3+H2O 2CH3CH2OH��O2
CH3COOCH2CH3+H2O 2CH3CH2OH��O2![]() 2CH3CHO��2H2O 3CH��CH
2CH3CHO��2H2O 3CH��CH![]()
![]() �ӳɷ�Ӧ cd
�ӳɷ�Ӧ cd
��������
��̬��A�ڱ�״���µ��ܶ���1.16 g��L1��M =22.4��= 22.4L��mol1��1.16 g��L1= 26g/mol��B�IJ���������������һ������ʯ�ͻ�����չˮƽ��˵��BΪCH2=CH2����AΪHC��CH��CΪCH3CH2OH��C(CH3CH2OH)��������ΪD(CH3CHO)��D(CH3CHO)��������ΪE(CH3COOH)��������Ҵ�������Ӧ����F(CH3COOCH2CH3)��G��һ�ָ߷��ӻ������GΪ����ϩ��
(1)DΪCH3CHO��������������ȩ����B����ϩ����ṹʽΪ![]() ���ʴ�Ϊ��ȩ����
���ʴ�Ϊ��ȩ����![]() ��
��
(2)C+E��F��������Ҵ�����������Ӧ���䷴Ӧ�Ļ�ѧ����ʽCH3CH2OH+ CH3COOH![]() CH3COOCH2CH3+H2O���ʴ�Ϊ��CH3CH2OH+ CH3COOH
CH3COOCH2CH3+H2O���ʴ�Ϊ��CH3CH2OH+ CH3COOH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
(3)C��D���Ҵ���������Ϊ��ȩ���䷴Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH��O2![]() 2CH3CHO��2H2O���ʴ�Ϊ��2CH3CH2OH��O2
2CH3CHO��2H2O���ʴ�Ϊ��2CH3CH2OH��O2![]() 2CH3CHO��2H2O��
2CH3CHO��2H2O��
(4)��֪������A��һ�������¿ɺϳɲ���ʹ����KMnO4��Һ��ɫ���л�����ݺϳɷ�Ӧ���ص��ԭ���غ�õ����л���Ϊ�����úϳɷ�Ӧ�Ļ�ѧ����ʽ3CH��CH![]()
![]() ����Ӧ����Ϊ�ӳɷ�Ӧ���ʴ�Ϊ��3CH��CH
����Ӧ����Ϊ�ӳɷ�Ӧ���ʴ�Ϊ��3CH��CH![]()
![]() ���ӳɷ�Ӧ��
���ӳɷ�Ӧ��
(5)a. E(CH3COOH)�ǵ���ʣ���a����
b. A(HC��CH)������ԭ�Ӵ���ͬһֱ���ϣ���b����
c. ����ʱ��D(CH3CHO)��������������ͭ����Һ��Ӧ����ש��ɫ��������c��ȷ��
d. 75%(�������)���Ҵ�ˮ��Һ������ҽ����������d��ȷ��
e. ���̶���С���ƿ�Ͷ���Ҵ��У��ƿ���Һ��ײ�������ð���ݣ���e����
��������������cd��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ���Ȼ��ƾ���Ͷ�����̼����Ľṹʾ��ͼ���������־���˵����ȷ����
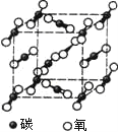
A.���־����ھ����й��ۼ�B.�������־����������ԭ��
C.���־�����������Ӿ���D.���ߵ�Ӳ�ȡ��۷е���ϴ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��EΪԪ�����ڱ���ԭ��������������Ķ���������Ԫ�أ�X��Y��Z�ֱ�ΪB��C��E��Ӧ�ĵ��ʣ��ס��� ��������Ϊ���в���Ԫ����ɵĻ��������C��Dͬ���壬����һ����ʹʪ��ĺ�ɫʯ����ֽ���������壬�����ڼ���Z�����Ƿ�й©���������ʼ��ת����ϵ��ͼ��ʾ��

��1��B��C��D��E����Ԫ���γɵļ������У����Ӱ뾶��С�����˳����___(�û�ѧ�����ʾ����
��2��������ʵ��˵��DԪ�صķǽ�����С��E����___
a.��Ӧ�⻯��Ļ�ԭ�ԣ�D��E
b.D��E��Ԫ�صļ��⻯�����ȷֽ⣬���ߵķֽ��¶ȸ�
c.���ʵķе㣺D��E
��3����֪Ԫ��Tsλ�ڵ������ڣ���Eͬ�壬д����������Ϊ176�ĺ��ط���___��Ԥ��Ts���仯��������ܾ��е�������___��
a.����������ijЩ�л��ܼ� b.��������ɫ����
c.�⻯����ȶ� d.�������ӿ���Ag+�γ�������
��4��A��B����Ԫ�ؿ����һ�����ӻ�����BA5���û�����ĵ���ʽΪ___��
��5��A��B��D����Ԫ��������ӻ�����W����֪��
��1molW������������������Һ���ȣ�����22.4L����(��״��)��
��W�������ᷴӦ����һ�ֳ�������ζ�����塣��W��___��д���ٵ����ӷ���ʽ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס��ҡ����������������У��ס��ҡ�����������ͬ��ij��Ԫ�أ�����֮���ת����ϵ��ͼ��ʾ�������й����ʵ��ƶ���ȷ���ǣ� ��
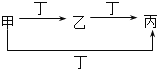
�� | �� | �� | �� | |
A | S | SO2 | SO3 | O2 |
B | CO32- | HCO3- | CO2 | H+ |
C | Cl2 | FeCl3 | FeCl2 | Fe |
D | Al3+ | Al��OH��3 | AlO2- | NH3H2O |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������廯����Q����Է�������Ϊ148��Q����ʹ������Ȼ�̼��Һ��ɫ��������С�մ�Ӧ�ų����塣�����й�Q�������������
A. ����ʽΪC9H8O2 B. ����������Ӧ
C. �ṹ��ʽ��5�� D. ����������ԭ�Ӳ��ܴ���ͬһƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2-����1��3������ϩ��һ����Ҫ�Ļ���ԭ�ϡ����Է������·�Ӧ����֪��
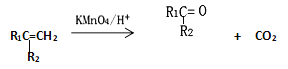
![]()
![]()
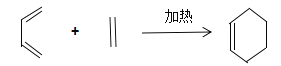

��ش��������⣺
(1)2-����1��3������ϩ��������������ˮ�ȷ����ӳɷ�Ӧ��
����ȫ�������ӳ������л����������____________��
������ˮ����1��2���ӳ������л�����Ľṹ��ʽΪ____________ �� _____________��
������ˮ����1��4���ӳɷ�Ӧ�Ļ�ѧ����ʽΪ_______________________________________��
(2)BΪ������Ԫ�����л��д��2-����1��3������ϩ����ϩ��Ӧ�Ļ�ѧ����ʽ________________________________________��
(3)Y(![]() )����Ȼ����Ҫ�ɷ֡��ܷ����ķ�Ӧ��_________(����)��
)����Ȼ����Ҫ�ɷ֡��ܷ����ķ�Ӧ��_________(����)��
A���ӳɷ�Ӧ B.������Ӧ C.������Ӧ
(4)X�ķ���ʽΪC3H4O3����ṹ��ʽΪ_________________��X���Ҵ�����������Ӧ�Ļ�ѧ����ʽ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶��£���2L�ܱ������г���4molA�����3molB���壬�������з�Ӧ��2A(g)+B(g)![]() C(g)+xD(g)��5s�ﵽƽ�⡣�ﵽƽ��ʱ��������1molC���ⶨD��Ũ��Ϊ1mol/L��
C(g)+xD(g)��5s�ﵽƽ�⡣�ﵽƽ��ʱ��������1molC���ⶨD��Ũ��Ϊ1mol/L��
(1)��x=__��
(2)�����ʱ��A��ƽ����Ӧ����Ϊ___��
(3)ƽ��ʱB��Ũ��Ϊ___��
(4)A��ת����Ϊ___��
(5)����������˵��������Ӧ�ﵽƽ��״̬����___��
A.��λʱ����ÿ����2molA��ͬʱ����1molC
B.��λʱ����ÿ����1molB��ͬʱ����1molC
C.D������������ٱ仯
D.���������ڻ�������ѹǿ���ٱ仯
E.B��C��Ũ��֮��Ϊ1��1
(6)ij�¶������ܱ������У�X��Y��Z������̬���ʵ����ʵ�����ʱ��仯������ͼ���÷�Ӧ�Ļ�ѧ����ʽ��___��
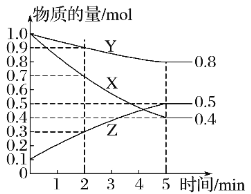
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������������Ҵ���Ũ������������ȡ����������װ����ͼ������˵����ȷ����
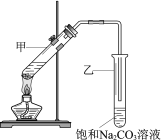
A. ����Թ����ȼ�Ũ���ᣬ�ټ��Ҵ�������
B. ���Թ��е��ܲ�����Һ���£���Ϊ�˷�ֹ����
C. ����������ᣬ��ʹ�Ҵ���ȫת��Ϊ��������
D. ʵ����ϣ��ɽ����������ӻ�����й��˳���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���йش����Ĵ�����������ɴ����Ҵ�������ʵ�����õ�һЩ��ʶ����ʵ��װ����ͼ��ʾ��ʵ�����Ϊ��Ԥ��ʹ���Ž��Ҵ���������ͼװ�úã���ͭ˿���м䲿�ּ��ȣ�Ƭ�̺�ʼ�н��ࣨ��Ъ�ԣ��ع�����������ɹ۲쵽���Ե�ʵ������
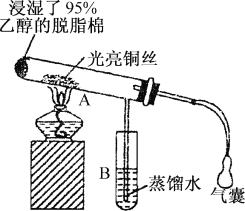
��ش��������⣺
��1�������ȵ�ͭ˿��������Ӧ�Ļ�ѧ����ʽΪ��________��
��2����A���пɹ۲쵽_______��ʵ�������п���ʶ���ڸ�ʵ������У������������ʱ�μ��˻�ѧ��Ӧ��������ʶ�������������ʱ��Ҫһ����_______��
��3����֪����������Ӧ�Ƿ��ȷ�Ӧ����ʵ�����һ��ʱ������ֻ�����ƾ��ƣ���Ӧ���ܷ�������У�______��ԭ���ȵ�ͭ˿����ʲô����_______��
��4����֤�Ҵ���������Ļ�ѧ������_______��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com