
Ä³Ń§ÉśÓĆŅŃÖŖĪļÖŹµÄĮæÅØ¶ČµÄŃĪĖįĄ“²ā¶ØĪ“ÖŖĪļÖŹµÄĮæÅØ¶ČµÄNaOHČÜŅŗŹ±£¬Ń”Ōń¼×»ł³Č×÷ÖøŹ¾¼Į”£ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
(1)ÓƱź×¼µÄŃĪĖįµĪ¶Ø“ż²āµÄNaOHČÜŅŗŹ±£¬×óŹÖĪÕĖįŹ½µĪ¶Ø¹ÜµÄ»īČū£¬ÓŅŹÖŅ”¶Æ׶ŠĪĘ棬ŃŪ¾¦×¢ŹÓ________£¬Ö±µ½Ņņ¼ÓČėŅ»µĪŃĪĖįŗó£¬ČÜŅŗÓÉ»ĘÉ«±äĪŖ³ČÉ«£¬²¢________ĪŖÖ¹”£
(2)ĻĀĮŠ²Ł×÷ÖŠæÉÄÜŹ¹Ėł²āNaOHČÜŅŗµÄÅØ¶ČŹżÖµĘ«µĶµÄŹĒ________(Ģī×ÖÄøŠņŗÅ)”£
A£®ĖįŹ½µĪ¶Ø¹ÜĪ“ÓƱź×¼ŃĪĖįČóĻ“¾ĶÖ±½Ó×¢Čė±ź×¼ŃĪĖį
B£®µĪ¶ØĒ°Ź¢·ÅNaOHČÜŅŗµÄ׶ŠĪĘæÓĆÕōĮóĖ®Ļ“¾»ŗóƻӊøÉŌļ
C£®ĖįŹ½µĪ¶Ø¹ÜŌŚµĪ¶ØĒ°ÓŠĘųÅŻ£¬µĪ¶ØŗóĘųÅŻĻūŹ§
D£®¶ĮČ”ŃĪĖįĢå»żŹ±£¬æŖŹ¼ŃöŹÓ¶ĮŹż£¬µĪ¶Ø½įŹųŹ±ø©ŹÓ¶ĮŹż
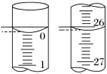
(3)ČōµĪ¶ØæŖŹ¼ŗĶ½įŹųŹ±£¬ĖįŹ½µĪ¶Ø¹ÜÖŠµÄŅŗĆęČēĶ¼ĖłŹ¾£¬ŌņĘšŹ¼¶ĮŹżĪŖ______mL£¬ÖÕµć¶ĮŹżĪŖ______mL£¬ĖłÓĆŃĪĖįČÜŅŗµÄĢå»żĪŖ______mL”£
(4)Ä³Ń§Éśøł¾Ż3“ĪŹµŃé·Ö±š¼ĒĀ¼ÓŠ¹ŲŹż¾ŻČēĻĀ±ķ£ŗ
| µĪ¶Ø“ĪŹż | “ż²āNaOHČÜŅŗµÄĢå»ż/mL | 0.100 0 mol·L£1ŃĪĖįµÄĢå»ż/mL | ||
| µĪ¶ØĒ°æĢ¶Č | µĪ¶ØŗóæĢ¶Č | ČÜŅŗĢå»ż/mL | ||
| µŚŅ»“Ī | 25.00 | 0.00 | 26.11 | 26.11 |
| µŚ¶ž“Ī | 25.00 | 1.56 | 30.30 | 28.74 |
| µŚČż“Ī | 25.00 | 0.22 | 26.31 | 26.09 |
ŅĄ¾ŻÉĻ±ķŹż¾ŻĮŠŹ½¼ĘĖćøĆNaOHČÜŅŗµÄĪļÖŹµÄĮæÅØ¶Č”£
“š°ø””(1)׶ŠĪĘæÖŠČÜŅŗŃÕÉ«±ä»Æ””ŌŚ°ė·ÖÖÓÄŚ²»±äÉ«””(2)D””(3)0.00””26.10””26.10
(4)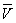 £½
£½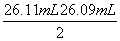 £½26.10 mL£¬c(NaOH)£½
£½26.10 mL£¬c(NaOH)£½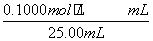 £½0.104 4 mol·L£1
£½0.104 4 mol·L£1
½āĪö””ŌŚĒóc(NaOH)ŗĶ½ųŠŠĪó²ī·ÖĪöŹ±Ó¦ŅĄ¾Ż¹«Ź½£ŗc(NaOH)£½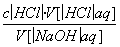 ”£ÓūĒóc(NaOH)£¬ŠėĻČĒóV[(HCl)aq]ŌŁ“śČė¹«Ź½£»½ųŠŠĪó²ī·ÖĪöŹ±£¬ŅŖæ¼ĀĒŹµ¼Ź²Ł×÷¶ŌĆæŅ»øöĮ漓V[(HCl)aq]ŗĶV[(NaOH)aq]µÄÓ°Ļģ£¬½ų¶ųÓ°Ļģc(NaOH)”£
”£ÓūĒóc(NaOH)£¬ŠėĻČĒóV[(HCl)aq]ŌŁ“śČė¹«Ź½£»½ųŠŠĪó²ī·ÖĪöŹ±£¬ŅŖæ¼ĀĒŹµ¼Ź²Ł×÷¶ŌĆæŅ»øöĮ漓V[(HCl)aq]ŗĶV[(NaOH)aq]µÄÓ°Ļģ£¬½ų¶ųÓ°Ļģc(NaOH)”£
(1)æ¼²éĖį¼īÖŠŗĶµĪ¶ØŹµŃéµÄ¹ę·¶²Ł×÷”£
(2)æ¼²éÓÉÓŚ²»ÕżČ·²Ł×÷ŅżĘšµÄĪó²ī·ÖĪö”£µĪ¶Ø¹ÜĪ“ÓƱź×¼ŃĪĖįČóĻ“£¬ÄŚ±Śø½×ÅŅ»²ćĖ®£¬æɽ«¼ÓČėµÄŃĪĖįĻ”ŹĶ£¬ĻūŗÄĻąĶ¬ĮæµÄ¼ī£¬ĖłŠčŃĪĖįµÄĢå»żĘ«“󣬽į¹ūĘ«øߣ»ÓĆ¼īŹ½µĪ¶Ø¹ÜČ”³öµÄ“ż²āNaOHČÜŅŗµÄĪļÖŹµÄĮæŅ»µ©Č·¶Ø£¬µ¹Čė׶ŠĪĘæŗó£¬Ė®µÄ¼ÓČė²»Ó°ĻģOH£µÄĪļÖŹµÄĮ棬Ņ²¾Ķ²»Ó°Ļģ½į¹ū£»ČōÅųöĘųÅŻ£¬ŅŗĆę»įĻĀ½µ£¬¹Ź¶ĮČ”VĖįĘ«“󣬽į¹ūĘ«øߣ»ÕżČ·¶ĮŹż(ŠéĻß²æ·Ö)ŗĶ“ķĪó¶ĮŹż(ŹµĻß²æ·Ö)ČēĶ¼ĖłŹ¾£ŗ
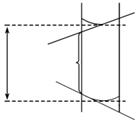
(3)¶ĮŹżŹ±£¬ŅŌ°¼ŅŗĆęµÄ×īµĶµćĪŖ»ł×¼”£
(4)ĻČĖć³öŗÄÓƱź×¼ŃĪĖįµÄĘ½¾łÖµ
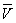 £½
£½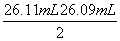 £½26.10 mL(µŚ¶ž“ĪĘ«²īĢ«“ó£¬ÉįČ„)£¬
£½26.10 mL(µŚ¶ž“ĪĘ«²īĢ«“ó£¬ÉįČ„)£¬
c(NaOH)£½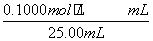 £½0.104 4 mol·L£1”£
£½0.104 4 mol·L£1”£
””


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠÓŠ¹ŲĄė×Ó¼üµÄŠšŹöÖŠÕżČ·µÄŹĒ(””””)
A£®Ąė×Ó»ÆŗĻĪļÖŠÖ»ŗ¬ÓŠĄė×Ó¼ü
B£®¹²¼Ū»ÆŗĻĪļÖŠæÉÄÜŗ¬Ąė×Ó¼ü
C£®ŗ¬Ąė×Ó¼üµÄ»ÆŗĻĪļ²»Ņ»¶ØĪŖĄė×Ó»ÆŗĻĪļ
D£®¹²¼Ū»ÆŗĻĪļÖŠ²»ŗ¬Ąė×Ó¼ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ō×Ó¼äŠĪ³É·Ö×ÓŹ±£¬¾ö¶Øø÷Ō×ÓĻą»„½įŗĻµÄŹżĮæ¹ŲĻµµÄŹĒ(””””)
A£®¹²¼Ū¼üµÄ·½ĻņŠŌ B£®¹²¼Ū¼üµÄ±„ŗĶŠŌ
C£®¹²¼Ū¼üŌ×ÓµÄ“óŠ” D£®¹²¼Ū¼üµÄĪČ¶ØŠŌ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
1 mL pH£½9µÄNaOHČÜŅŗ£¬¼ÓĖ®Ļ”ŹĶµ½10 mL£¬pH£½________£»¼ÓĖ®Ļ”ŹĶµ½100 mL£¬pH________7”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
KMnO4(H£«)ČÜŅŗ”¢äåĖ®”¢Na2CO3ČÜŅŗ”¢Ļ”ŃĪĖįÓ¦·Ö±šŹ¢·ÅŌŚÄÄÖÖµĪ¶Ø¹ÜÖŠ£æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĪŖČ·¶Ø¼ÓČėŠæ»Ņ(Ö÷ŅŖ³É·ÖĪŖZn”¢ZnO£¬ŌÓÖŹĪŖĢś¼°ĘäŃõ»ÆĪļ)µÄĮ棬ŹµŃéÖŠŠč²ā¶Ø³żČ„H2O2ŗóČÜŅŗÖŠCu2£«µÄŗ¬Į攣ŹµŃé²Ł×÷ĪŖ£ŗ×¼Č·ĮæČ”Ņ»¶ØĢå»żµÄŗ¬ÓŠCu2£«µÄČÜŅŗÓŚ“ųČū׶ŠĪĘæÖŠ£¬¼ÓŹŹĮæĖ®Ļ”ŹĶ£¬µ÷½ŚČÜŅŗpH£½3”«4£¬¼ÓČė¹żĮæµÄKI£¬ÓĆNa2S2O3±ź×¼ČÜŅŗµĪ¶ØÖĮÖÕµć”£ÉĻŹö¹ż³ĢÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ČēĻĀ£ŗ
2Cu2£«£«4I£===2CuI(°×É«)”ż£«I2
2S2O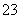 £«I2===2I££«S4O
£«I2===2I££«S4O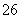
¢ŁµĪ¶ØŃ”ÓƵÄÖøŹ¾¼ĮĪŖ____________£¬µĪ¶ØÖÕµć¹Ū²ģµ½µÄĻÖĻóĪŖ__________________”£
¢ŚČōµĪ¶ØĒ°ČÜŅŗÖŠµÄH2O2ƻӊ³ż¾”£¬Ėł²ā¶ØµÄCu2£«ŗ¬Į潫»į________(Ģī”°Ę«øß”±”¢”°Ę«µĶ”±»ņ”°²»±ä”±)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²ā¶ØNa2S2O3·5H2O²śĘ·“æ¶Č
×¼Č·³ĘČ”W g Na2S2O3·5H2O²śĘ·£¬ÓĆŹŹĮæÕōĮóĖ®Čܽā£¬ŅŌµķ·Ū×÷ÖøŹ¾¼Į£¬ÓĆ0.100 0 mol·L£1µāµÄ±ź×¼ČÜŅŗµĪ¶Ø”£
·“Ó¦ŌĄķĪŖ2S2O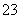 £«I2===S4O
£«I2===S4O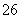 £«2I£
£«2I£

(5)µĪ¶ØÖĮÖÕµćŹ±£¬ČÜŅŗŃÕÉ«µÄ±ä»Æ£ŗ___________________________________________”£
(6)²ā¶ØĘšŹ¼ŗĶÖÕµćµÄŅŗĆęĪ»ÖĆČēĶ¼£¬ŌņĻūŗĵāµÄ±ź×¼ČÜŅŗĢå»żĪŖ________mL”£²śĘ·µÄ“æ¶ČĪŖ(ÉčNa2S2O3·5H2OĻą¶Ō·Ö×ÓÖŹĮæĪŖM)________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚøÜøĖµÄĮ½¶Ė·Ö±š¹Ņ×ÅÖŹĮæŗĶĢå»ż¶¼ĻąĶ¬µÄĀĮĒņŗĶĢśĒņ£¬“ĖŹ±øÜøĖĘ½ŗā”£Č»ŗó½«Į½Ēņ·Ö±š½žĆ»ŌŚĒāŃõ»ÆÄĘČÜŅŗŗĶĮņĖįĶČÜŅŗ֊ʬæĢ£¬ČēĶ¼£¬ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
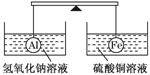
A£®Į½ÉÕ±ÖŠ¾łĪŽĘųÅŻ²śÉś
B£®×ó±ßÉÕ±ÖŠµÄČÜŅŗÖŹĮæ¼õÉŁĮĖ
C£®Č„µōĮ½ÉÕ±£¬øÜøĖČŌĘ½ŗā
D£®ÓŅ±ßĢśĒņÉĻ³öĻÖŗģÉ«
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¹żŃõ»ÆÄĘæÉ×÷ĪŖŃõĘųµÄĄ“Ō“”£³£ĪĀ³£Ń¹ĻĀ¶žŃõ»ÆĢ¼ŗĶ¹żŃõ»ÆÄĘ·“Ó¦ŗó£¬Čō¹ĢĢåÖŹĮæŌö¼ÓĮĖ28 g£¬·“Ó¦ÖŠÓŠ¹ŲĪļÖŹµÄĪļĄķĮæÕżČ·µÄŹĒ(NA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£Źż)(””””)
| Ń”Ļī | ¶žŃõ»ÆĢ¼ | Ģ¼ĖįÄĘ | ×ŖŅʵĵē×Ó |
| A | 1 mol | 2NA | |
| B | 22.4 L | 1 mol | |
| C | 106 g | 1 mol | |
| D | 106 g | 2NA |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com