
��֪CuSO4��Һ�ֱ���Na2CO3��Һ��Na2S��Һ�ķ�Ӧ������£�
��1��CuSO4 +Na2CO3 ��Ҫ��Cu2++ CO32�� + H2O == Cu(OH)2��+ CO2��
��Ҫ��Cu2+ + CO32��== CuCO3��
��2��CuSO4 + Na2S ��Ҫ��Cu2+ + S2��== CuS��
��Ҫ��Cu2+ + S2��+ 2H2O == Cu(OH)2��+H2S��
���м������ʵ��ܽ�ȴ�С�ıȽ��У���ȷ����
A��CuS <Cu(OH)2<CuCO3 B��CuS >Cu(OH)2>CuCO3
C��Cu(OH)2>CuCO3>CuS D��Cu(OH)2<CuCO3<CuS

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(16��)������Һ��һ�ֱ�����ɱ�������㷺Ӧ������ľ�������ͻ����ϣ�����ɫ�ĵ������������Ʋ�����Һ����Ҫԭ�ϡ���֪CuSO4��5H2O�IJ��ֽṹ�ɱ�ʾ���£�
��1��д��ͭԭ�Ӽ۵��Ӳ�ĵ����Ų�ʽ____________����ͭͬ���ڵ�����Ԫ�صĻ�̬ԭ����������������ͭԭ����ͬ��Ԫ����__________(��Ԫ�ط���)��
��2��������ͼ�а�CuSO4��5H2O�ṹ�еĻ�ѧ���ö��ߡ���������ʾ������
��3����ŨCuSO4��Һ�м��������Ũ��NH3��H2Oֱ��ԭ�����ɵij���ǡ���ܽ�Ϊֹ���õ�����ɫ��Һ��С�ļ���Լ����Һ�������C2H5OH��ʹ֮�ֳ����㣬���á�����һ��ʱ���ɹ۲쵽�����㡰���紦���²���������ɫCu(NH3)4SO4��H2O���塣ʵ��������C2H5OH��������______________________________________________��
��4��Cu(NH3)4SO4��H2O�����г����������ԭ������______________���ӻ����������sp3��ԭ����____________________________��
��5���罫����ɫ��Һ���ȣ����ܵõ�ʲô�����________________________________��
��6����CoCl2�ܽ���ˮ��Ӱ�ˮֱ�������ɵ�Co(OH)2�������ܽ���ټӰ�ˮ��ʹ����[Co(NH3)6]2+����ʱ����Һ��ͨ��������õ��IJ�������һ������ɿ���CoCl3��5NH3��ʾ���ѷ������CoCl3��5NH3����ˮ����������������Һ��������AgCl���������ⶨ��ÿ1 mol CoCl3��5NH3ֻ����2 mol AgCl����д����ʾ�������ṹ�Ļ�ѧʽ���ܵ���λ��Ϊ6��___________����������е�Co���ϼ�Ϊ__ ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��֣��������ѧУ�߶���ѧ����ĩ���Ի�ѧ���� ���ͣ������
(16��)������Һ��һ�ֱ�����ɱ�������㷺Ӧ������ľ�������ͻ����ϣ�����ɫ�ĵ������������Ʋ�����Һ����Ҫԭ�ϡ���֪CuSO4��5H2O�IJ��ֽṹ�ɱ�ʾ���£�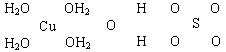
��1��д��ͭԭ�Ӽ۵��Ӳ�ĵ����Ų�ʽ____________����ͭͬ���ڵ�����Ԫ�صĻ�̬ԭ����������������ͭԭ����ͬ��Ԫ����__________(��Ԫ�ط���)��
��2��������ͼ�а�CuSO4��5H2O�ṹ�еĻ�ѧ���ö��ߡ���������ʾ������
��3����ŨCuSO4��Һ�м��������Ũ��NH3��H2Oֱ��ԭ�����ɵij���ǡ���ܽ�Ϊֹ���õ�����ɫ��Һ��С�ļ���Լ����Һ�������C2H5OH��ʹ֮�ֳ����㣬���á�����һ��ʱ���ɹ۲쵽�����㡰���紦���²���������ɫCu(NH3)4SO4��H2O���塣ʵ��������C2H5OH��������______________________________________________��
��4��Cu(NH3)4SO4��H2O�����г����������ԭ������______________���ӻ����������sp3��ԭ����____________________________��
��5���罫����ɫ��Һ���ȣ����ܵõ�ʲô�����________________________________��
��6����CoCl2�ܽ���ˮ��Ӱ�ˮֱ�������ɵ�Co(OH)2�������ܽ���ټӰ�ˮ��ʹ����[Co(NH3)6]2+����ʱ����Һ��ͨ��������õ��IJ�������һ������ɿ���CoCl3��5NH3��ʾ���ѷ������CoCl3��5NH3����ˮ����������������Һ��������AgCl���������ⶨ��ÿ1 mol CoCl3��5NH3ֻ����2 mol AgCl����д����ʾ�������ṹ�Ļ�ѧʽ���ܵ���λ��Ϊ6��___________����������е�Co���ϼ�Ϊ__ ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ���и�һ��ѧ�������ʼ����ۻ�ѧ�Ծ����������� ���ͣ�ʵ����
(12��)����д����ѧ�γ�����������ȡ�����Ļ�ѧ����ʽ��
�� ��
��.ʵ������ͨ����MnO2�������ֽ�������⣬��֪CuSO4��Һ�Թ�������ķֽ�Ҳ���д����ã�ijʵ����ȤС��ͬѧ������������ҺҲ�����������Ӧ����ͬ�������ã�����������������̽������������������ʵ�鱨�棺
(1)ʵ����̣���һ֧�Թ��м���5 mL 5%��H2O2��Һ��Ȼ�����������FeCl3��Һ���Ѵ����ǵ�ľ�������Թܡ�
ʵ������ ��
ʵ����ۣ�FeCl3��Һ���Դ��ֽ�H2O2��
(2)��֪FeCl3��ˮ�пɽ����Fe3����Cl����ͬѧ��������²��룺
��ͬѧ�IJ��룺�������ֽ�H2O2����FeCl3��Һ�е�H2O��
��ͬѧ�IJ��룺�������ֽ�H2O2����FeCl3��Һ�е�Fe3����
��ͬѧ�IJ��룺�������ֽ�H2O2����FeCl3��Һ�е�Cl����
����Ϊ����ܵ��� ͬѧ�IJ��룬������ ��
(3)ͬѧ�Ƕ����µ��������룬��ʵ�������̽��������¼����,������ϸ�����������
ʵ����� ʵ������ ����
| ʵ����� | ʵ������ | ���� |
| ��ʢ��5 mL 5%��H2O2��Һ���Թ��е��������� HCl���Ѵ����ǵ�ľ�������Թ�. | ���������� | |
| ��ʢ��5 mL 5%��H2O2��Һ���Թ��е���������Fe2(SO4)3���Ѵ����ǵ�ľ�������Թ�. | �Թ����д������ݲ����������ǵ�ľ����ȼ | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��㶫ʡ���и�һ��ѧ�������ʼ����ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
(12��)����д����ѧ�γ�����������ȡ�����Ļ�ѧ����ʽ��
�� ��
��.ʵ������ͨ����MnO2�������ֽ�������⣬��֪CuSO4��Һ�Թ�������ķֽ�Ҳ���д����ã�ijʵ����ȤС��ͬѧ������������ҺҲ�����������Ӧ����ͬ�������ã�����������������̽������������������ʵ�鱨�棺
(1)ʵ����̣���һ֧�Թ��м���5 mL 5%��H2O2��Һ��Ȼ�����������FeCl3��Һ���Ѵ����ǵ�ľ�������Թܡ�
ʵ������ ��
ʵ����ۣ�FeCl3��Һ���Դ��ֽ�H2O2��
(2)��֪FeCl3��ˮ�пɽ����Fe3����Cl����ͬѧ��������²��룺
��ͬѧ�IJ��룺�������ֽ�H2O2����FeCl3��Һ�е�H2O��
��ͬѧ�IJ��룺�������ֽ�H2O2����FeCl3��Һ�е�Fe3����
��ͬѧ�IJ��룺�������ֽ�H2O2����FeCl3��Һ�е�Cl����
����Ϊ����ܵ��� ͬѧ�IJ��룬������ ��
(3)ͬѧ�Ƕ����µ��������룬��ʵ�������̽��������¼����,������ϸ�����������
ʵ����� ʵ������ ����
|
ʵ����� |
ʵ������ |
���� |
|
��ʢ��5 mL 5%��H2O2��Һ���Թ��е��������� HCl���Ѵ����ǵ�ľ�������Թ�. |
���������� |
|
|
��ʢ��5 mL 5%��H2O2��Һ���Թ��е���������Fe2(SO4)3���Ѵ����ǵ�ľ�������Թ�. |
�Թ����д������ݲ����������ǵ�ľ����ȼ |
|
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com