
ÓŠŅ»ĘæĪŽÉ«³ĪĒåµÄČÜŅŗ£¬ĘäÖŠæÉÄÜŗ¬Na+”¢Mg2+”¢H+”¢Fe3+”¢CO32-”¢Cl-”¢Br-ÖŠµÄŅ»ÖÖ»ņ¼øÖÖ£¬Č”øĆČÜŅŗ½ųŠŠČēĻĀŹµŃé£ŗ
¢ŁÓĆPHŹŌÖ½¼ģŃ飬±ķĆ÷ČÜŅŗ³ŹĒæĖįŠŌ
¢ŚČ”²æ·ÖČÜŅŗ£¬¼ÓČėÉŁĮæµÄCCl4¼°ŹżµĪŠĀÖʵÄĀČĖ®£¬Õńµ“ŗóCCl4²ćĻŌ³ČŗģÉ«
¢Ū½«¢ŚµĆµ½µÄČÜŅŗµĪ¼ÓĻõĖįŅųČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É£¬µĪ¼ÓĻ”ĻõĖį³Įµķ²»Čܽā”£
¢ÜĮķČ”²æ·ÖČÜŅŗ£¬ÖšµĪ¼ÓČėĻ”NaOHČÜŅŗ£¬Ź¹ČÜŅŗ“ÓĖįŠŌÖš½„×Ŗ±äĪŖ¼īŠŌ£¬ŌŚµĪ¼Ó¹ż³ĢÖŠ¼°µĪ¼ÓĶź±Ļŗó£¬ČÜŅŗÖŠ¾łĪŽ³ĮµķÉś³É
øł¾ŻÉĻŹöŹµŃéŹĀŹµČ·¶Ø²¢»Ų“š£ŗ
£Ø1£©ŌŚČÜŅŗÖŠ£¬æĻ¶Ø“ęŌŚµÄĄė×ÓÓŠ ”£
£Ø2£©æĻ¶Ø²»“ęŌŚµÄĄė×ÓÓŠ ”£
£Ø3£©æÉÄÜ“ęŌŚµÄĄė×ÓÓŠ ”£
£Ø1£©H+ Br-
£Ø2£©Mg2+ Fe3+”¢CO32-
£Ø3£©Na+ Cl-
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©¢ŁČÜŅŗĪŽÉ«£¬ĖµĆ÷²»ŗ¬Fe3+£»ČÜŅŗ³ŹĒæĖįŠŌ£¬ĖµĆ÷ČÜŅŗÖŠ“ęŌŚH+£¬ĖłŅŌÓėĘä·“Ó¦µÄCO32-²»“ęŌŚ£»¢ŚµĪŠĀÖʵÄĀČĖ®£¬Õńµ“ŗóCCl4²ćĻŌ³ČŗģÉ«£¬ĖµĆ÷ČÜŅŗÖŠ“ęŌŚBr-£»¢Ū²»ÄÜÖ¤Ć÷ŌČÜŅŗÖŠÓŠCl-£¬ŅņĪŖÉĻ²½ÖŠµÄČÜŅŗĀČĖ®ÖŠŗ¬ĀČĄė×Ó¢ÜČÜŅŗÖŠ¾łĪŽ³ĮµķÉś³ÉĖµĆ÷ČÜŅŗÖŠ²»ŗ¬Mg2+£¬×ŪÉĻ·ÖĪöŌŚČÜŅŗÖŠ£¬æĻ¶Ø“ęŌŚµÄĄė×ÓÓŠH+ Br-
£Ø2£©æĻ¶Ø²»“ęŌŚµÄĄė×ÓÓŠMg2+ Fe3+”¢CO32-
£Ø3£©æÉÄÜ“ęŌŚµÄĄė×ÓÓŠNa+ Cl-
æ¼µć£ŗæ¼²éČÜŅŗÖŠĄė×Ó¼äµÄ·“Ó¦¼°Ąė×ÓŃÕÉ«µÄÅŠ¶Ļ



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
»ĘĶæóÖ÷ŅŖ³É·ÖŹĒ¶žĮņ»ÆŃĒĢśĶ£ØCuFeS2£©”£»ĘĶæó¾ČŪĮ¶”¢ģŃÉÕŗóµĆµ½“ÖĶŗĶĀÆŌü£¬Ņ±Į¶¹ż³ĢµÄÖ÷ŅŖ·“Ó¦ÓŠ£ŗ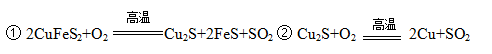
£Ø1£©¶žĮņ»ÆŃĒĢśĶŅ²æÉŅŌ±ķŹ¾ĪŖCuS”¤FeS£¬ĘäÖŠĮņŌŖĖŲµÄ»ÆŗĻ¼ŪŹĒ ”£
£Ø2£©·“Ó¦¢ŚÖŠ»¹Ō¼ĮŹĒ ”£
£Ø3£©Ä³Š£Ń§Ļ°Š”×éÓĆĮ¶Ķ²śÉśµÄĀÆŌü£Øŗ¬Fe2O3”¢FeO”¢SiO2”¢Al2O3µČ£©ÖʱøĢśŗģ£¬½ųŠŠČēĻĀŹµŃ锣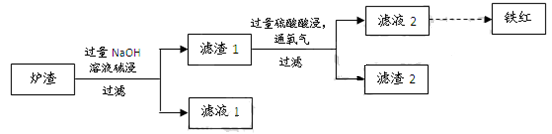
¢Ł ĀÆŌü¼ī½žŹ±·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ ”¢ ”£
¢Ś ĀĖŌü1ÖŠ¼ÓČėĮņĖį²¢ĶØČėŃõĘųæÉŹ¹FeO×Ŗ»ÆĪŖFe3+£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ £»ĪŖ¼ģŃéĢśŌŖĖŲŹĒ·ń±»Ńõ»ÆĶźČ«£¬Ó¦½ųŠŠµÄŹµŃéŹĒ£ŗȔɣĮæĀĖŅŗ2ÓŚŹŌ¹ÜÖŠ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
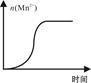
KMnO4ĖįŠŌČÜŅŗÓė²ŻĖį£ØH2C2O4£©ČÜŅŗ·“Ó¦Ź±£¬ČÜŅŗ×ĻÉ«»įÖš½„ĶŹČ„”£Ä³Ģ½¾æŠ”×éÓĆ²ā¶Ø“Ė·“Ó¦ČÜŅŗ×ĻÉ«ĻūŹ§ĖłŠčŹ±¼äµÄ·½·Ø£¬ŃŠ¾æĶā½ēĢõ¼ž¶Ō·“Ó¦ĖŁĀŹµÄÓ°Ļģ”£øĆŹµŃéĢõ¼ž×÷ČēĻĀĻŽ¶Ø£ŗ
¢ŁĖłÓĆKMnO4ĖįŠŌČÜŅŗµÄÅضČæÉŃ”Ōń£ŗ0.02 mol”¤L-1”¢0.002 mol”¤L-1£»
¢ŚĖłÓĆH2C2O4ČÜŅŗµÄÅضČæÉŃ”Ōń£ŗ0.2 mol”¤L-1”¢0.4 mol”¤L-1£»
¢ŪĆæ“ĪŹµŃ鏱KMnO4ĖįŠŌČÜŅŗµÄÓĆĮæ¾łĪŖ4 mL”¢H2C2O4ČÜŅŗµÄÓĆĮæ¾łĪŖ2mL”£
£Ø1£©ČōŅŖĢ½¾æ·“Ó¦ĪļÅØ¶Č”¢ĪĀ¶Č”¢“߻ƼĮ¶Ō·“Ó¦ĖŁĀŹµÄÓ°Ļģ£¬Ķعż±ä»»ÕāŠ©ŹµŃéĢõ¼ž£¬ÖĮÉŁŠčŅŖĶź³É____ ×鏵Ńé½ųŠŠ¶Ō±Č¼“æÉµĆ³ö½įĀŪ”£
£Ø2£©ŌŚĘäĖüĢõ¼žĻąĶ¬µÄĒéæöĻĀ£¬Ä³Ķ¬Ń§øıäKMnO4ĖįŠŌČÜŅŗµÄÅØ¶Č£¬²āµĆŹµŃ鏿¾Ż£Ø“Ó»ģŗĻÕńµ“¾łŌČæŖŹ¼¼ĘŹ±£©ČēĻĀ±ķĖłŹ¾£ŗ
| KMnO4ĖįŠŌČÜŅŗÅØ¶Č £Ømol”¤L-1£© | ČÜŅŗĶŹÉ«ĖłŠčŹ±¼ä£Ømin£© | | ||
| µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī | ||
| 0.02 | 14 | 13 | 11 | |
| 0.002 | 6.7 | 6.6 | 6.7 | |
| KMnO4ĖįŠŌČÜŅŗ | H2C2O4ČÜŅŗ | ||
| ÅضČ/ mol/L | Ģå»ż(ml) | ÅضČ/ mol/L | Ģå»ż£Øml£© |
| 0.02 | 2 | b | 4 |
| a | 2 | c | 4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ij¹¤³§ÓĆČķĆĢæó£Øŗ¬MnO2Ō¼70%¼°Al2 O3£©ŗĶÉĮŠææó£Øŗ¬ZnSŌ¼80%¼°FeS£©¹²Ķ¬Éś²śMnO2£¬ŗĶZn£Øøɵē³ŲŌĮĻ£©”£Į÷³ĢČēĻĀ£ŗ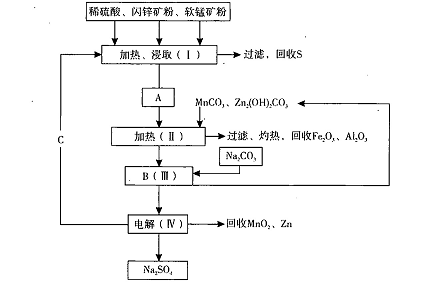
ŅŃÖŖ£ŗ¢ŁAŹĒMnSO4”¢ZnSO4”¢Fe2(SO4)3£¬Al2(SO4)3µÄ»ģŗĻŅŗ”£
¢ŚIVÖŠµÄµē½ā·“Ó¦Ź½ĪŖMnSO4+ZnSO4+2H2O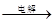 MnO2+Zn +2H2SO4”£
MnO2+Zn +2H2SO4ӣ
£Ø1£©AÖŠŹōÓŚ»¹Ō²śĪļµÄŹĒ ”£
£Ø2£©¼ÓČėMnCO3”¢Zn2(OH)2CO3µÄ×÷ÓĆŹĒ £ŗCµÄ»ÆѧŹ½ŹĒ ”£
£Ø3£©øĆÉś²śÖŠ³żµĆµ½Na2SO4”¢SµČø±²śĘ·Ķā£¬»¹æɵƵ½µÄø±²śĘ·ŹĒ ”£
£Ø4£©ø±²śĘ·SæÉÓĆÓŚÖĘĮņĖį£¬×Ŗ»Æ¹ż³ĢŹĒ£ŗS”śSO2”śSO3”śH2SO4”£Š“³öµŚ¶ž²½×Ŗ»ÆµÄ»Æѧ·½³ĢŹ½ ”£
£Ø5£©ŅŖ“ÓNa2SO4ČÜŅŗÖŠµĆµ½Ć¢Ļõ£Ø Na2SO4£®10H2O£©£¬Šč½ųŠŠµÄ²Ł×÷ÓŠÕō·¢ÅØĖõ”¢ ”¢
¹żĀĖ”¢Ļ“µÓ”¢øÉŌļµČ”£
£Ø6£©“ÓÉś²śMnO2ŗĶZnµÄ½Ē¶Č¼ĘĖć£¬ČķĆĢæóŗĶÉĮŠææóĶ¶ĮĻµÄÖŹĮæ±Č“óŌ¼ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(““ŠĀŌ¤²āĢā)(1)¢ŁŌŚµķ·Ūµā»Æ¼ŲČÜŅŗÖŠ£¬µĪ¼ÓÉŁĮæ“ĪĀČĖįÄĘ¼īŠŌČÜŅŗ£¬Į¢¼“»į擵½ČÜŅŗ±äĄ¶É«£¬ÕāŹĒŅņĪŖ________£¬Ąė×Ó·½³ĢŹ½ĪŖ__________________________”£
¢ŚŌŚµāŗĶµķ·ŪŠĪ³ÉµÄĄ¶É«ČÜŅŗÖŠ£¬µĪ¼ÓŃĒĮņĖįÄĘ¼īŠŌČÜŅŗ£¬·¢ĻÖĄ¶É«Öš½„ĻūŹ§£¬ÕāŹĒŅņĪŖ______________________________”£Ąė×Ó·½³ĢŹ½ŹĒ_______________________________”£
¢Ū¶Ō±Č¢ŁŗĶ¢ŚŹµŃéĖłµĆµÄ½į¹ū£¬½«I2”¢ClO£”¢SO42”ŖŠŌÓÉĒæµ½ČõµÄĖ³ŠņÅÅĮŠĪŖ_____________________________”£
(2)½ńÓŠĢśĘ¬”¢Ķʬ£¬Éč¼ĘŹµŃéÖ¤Ć÷ŅŌĻĀŹĀŹµ£¬²¢Š“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½”£
¢ŁÅØĮņĖįµÄŃõ»ÆŠŌ±ČĻ”ĮņĖįĒ攣________________________________”£
¢ŚĀČ»ÆĢśČÜŅŗÖŠFe3£«µÄŃõ»ÆŠŌ±ČĮņĖįĶČÜŅŗÖŠµÄCu2£«Ē攣__________________________”£
¢ŪĢśµÄ»¹ŌŠŌ±ČĶĒ攣
___________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŗ¬ÓŠÅ©Ņ©”¢Č¾ĮĻ”¢·Ó”¢Ēč»ÆĪļ£¬ŅŌ¼°ŅżĘšÉ«¶Č”¢³ōĪ¶µÄ·ĻĖ®£¬³£ÓĆ»ÆѧŃõ»Æ·Ø½ųŠŠ“¦Ąķ£¬ĖłÓƵÄŃõ»Æ¼ĮÓŠĀČĄą£ØČēŅŗĀČ”¢“ĪĀČĖįøĘ”¢“ĪĀČĖįÄĘµČ£©ŗĶŃõĄą£ØČēæÕĘų”¢³ōŃõ”¢¹żŃõ»ÆĒā”¢øßĆĢĖį¼ŲµČ£©”£Ņ»øöµäŠĶŹµĄżŹĒÓĆĀČŃõ»Æ·Ø“¦Ąķŗ¬ÓŠ¾ē¶¾µÄĒč»ÆĪļ£Øŗ¬CN££©µÄ·ĻĖ®”£ŌŚ¼īŠŌĢõ¼žĻĀ£ØpH£½8.5”«11£©£¬ĀČĘųæɽ«Ēč»ÆĪļÖŠCN£Ńõ»ÆĪŖÖ»ÓŠĖü¶¾ŠŌ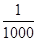 µÄĒčĖįŃĪ£Øŗ¬CNO££©”£
µÄĒčĖįŃĪ£Øŗ¬CNO££©”£
£Ø1£©Š“³öŗ¬CN£·ĻĖ®ÓĆĀČĘųŃõ»ÆÉś³ÉĒčĖįŃĪµÄĄė×Ó·½³ĢŹ½£ŗ________________________________________________________________”£
£Ø2£©ČōĻņŗ¬CNO£µÄ·ĻĖ®ÖŠŌŁĶØČėĀČĘų£¬æÉŹ¹CNO£×Ŗ»ÆĪŖĪŽ¶¾µÄĘųĢ壬Š“³öÕāøö·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ______________________________________________”£
£Ø3£©ŌŚÓĆŅŗĀČ²»±ćµÄµŲĒų£¬æÉÓĆĘÆ°×·Ū“¦Ąķŗ¬CN£µÄ·ĻĖ®£¬Čō½«ĘäŃõ»ÆĪŖCNO££¬ĘäĄė×Ó·½³ĢŹ½ĪŖ__________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijČÜŅŗæÉÄÜŗ¬ÓŠNa£«”¢K£«”¢Mg2£«”¢Cu2£«µČŃōĄė×Ó¼°MnO4”Ŗ”¢SiO32”Ŗ”¢AlO2”Ŗ”¢CO32”Ŗ”¢HCO3”Ŗ”¢SO42”Ŗ”¢Cl£µČŅõĄė×Ó£¬ŅŃÖŖ£ŗ¢ŁøĆČÜŅŗ³ŹĪŽÉ«£»¢Ś¾²ā¶ØČÜŅŗµÄpH£½12£»¢ŪȔɣĮæČÜŅŗ£¬¼ÓČė100 mL 2 mol”¤L£1Ļ”ŃĪĖį½ųŠŠĖį»Æ£¬ÓŠ°×É«³ĮµķÉś³É£¬»¹µĆµ½Ņ»ÖÖĪŽÉ«ĪŽĪ¶µÄĘųĢ壬øĆĘųĢåŹ¹³ĪĒåŹÆ»ŅĖ®(×ćĮæ)±ä»ė×Ē”£¶ŌĖį»ÆŗóµÄČÜŅŗ¹żĀĖ£¬µĆµ½ĀĖŅŗ¼×”£
(1)ÓÉ¢Ł¢Ś¢ŪæÉÅŠ¶Ļ£ŗŌČÜŅŗÖŠŅ»¶Ø²»“ęŌŚµÄĄė×ÓŹĒ________£¬Ņ»¶Ø“ęŌŚµÄĄė×ÓŹĒ________”£
(2)½«ĀĖŅŗ¼×·Ö³ÉĮ½µČ·Ż£¬Ņ»·ŻÖŠÖšµĪ¼ÓČė°±Ė®”¢×īÖÕÓŠ°×É«½ŗד³Įµķ£¬ĖµĆ÷ŌČÜŅŗÖŠŅ»¶ØÓŠ________(ĢīĄė×Ó·ūŗÅ)£¬øÕæŖŹ¼¼ÓČė°±Ė®Ź±£¬Ć»ÓŠ³Įµķ²śÉś£¬ŌŅņŹĒ____________________________________(ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾)£»ĮķŅ»·ŻÖŠ¼ÓČė×ćĮæµÄBa(NO3)2ČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É£¬ĖµĆ÷ŌČÜŅŗÖŠŅ»¶ØÓŠ________(ĢīĄė×Ó·ūŗÅ)£¬¹żĀĖµĆµ½ĀĖŅŗŅŅ”£
(3)ĶłĀĖŅŗŅŅÖŠ¼ÓČė×ćĮæµÄAgNO3ČÜŅŗ£¬¹żĀĖ”¢Ļ“µÓ”¢øÉŌļµĆ¹ĢĢå26.5 g£¬ŌņŌČÜŅŗÖŠŹĒ·ńÓŠCl££æ________(Ģī”°ŹĒ”±»ņ”°·ń”±)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŃÖŖ 0.4 mol ŅŗĢ¬ėĀŗĶ×ćĮæH2O2·“Ӧɜ³ÉµŖĘųŗĶĖ®ÕōĘųŹ±·Å³ö256.64 kJµÄČČĮ攣
(1)Š“³öėĀŗĶH2O2·“Ó¦µÄČČ»Æѧ·½³ĢŹ½: ”£
(2)ŅŃÖŖH2O(l)=H2O(g) ¦¤H="+44" kJ/mol,Ōņ16 gŅŗĢ¬ėĀÓė×ćĮæĖ«ŃõĖ®·“Ӧɜ³ÉµŖĘųŗĶŅŗĢ¬Ė®Ź±,·Å³öµÄČČĮæŹĒ ”£
(3)ÉĻŹö·“Ó¦Ó¦ÓĆÓŚ»š¼żĶĘ½ųĘ÷,³żŹĶ·Å³ö“óĮæČČĮæŗĶæģĖŁ²śÉś“óĮæĘųĢåĶā,»¹ÓŠŅ»øöŗÜĶ»³öµÄÓŵćŹĒ ”£
(4)Ļņ“ĪĀČĖįÄĘČÜŅŗÖŠĶØČėŅ»¶ØĪļÖŹµÄĮæµÄ°±ĘųæÉÉś³ÉėĀ,Š“³ö·“Ó¦µÄĄė×Ó·½³ĢŹ½: ,øĆ·“Ó¦µÄ»¹Ō²śĪļŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
½ųŠŠĪŪĖ®“¦Ąķ·ÖĪöŹ±,³£ÓĆĖ«Įņėź(H2Dz,¶žŌŖČõĖį)°Ń½šŹōĄė×ÓĀēŗĻ³ÉµēÖŠŠŌµÄĪļÖŹ,ŌŁÓĆCCl4ŻĶČ”ĀēŗĻĪļ,“Ó¶ų°Ń½šŹōĄė×Ó“ÓĖ®ČÜŅŗÖŠĶźČ«·ÖĄė³öĄ“”£ČēÓĆĖ«Įņėź(H2Dz)~CCl4·ÖĄėĪŪĖ®ÖŠµÄCu2+Ź±,ĻČ·¢ÉśĀēŗĻ·“Ó¦:Cu2++2H2Dz Cu(HDz)2+2H+,ŌŁ¼ÓČėCCl4,Cu(HDz)2¾ĶŗÜČŻŅ×±»ŻĶČ”µ½CCl4ÖŠ”£
Cu(HDz)2+2H+,ŌŁ¼ÓČėCCl4,Cu(HDz)2¾ĶŗÜČŻŅ×±»ŻĶČ”µ½CCl4ÖŠ”£
(1)Š“³öĖ«ĮņėźŗĶFe3+ĀēŗĻµÄĄė×Ó·½³ĢŹ½”” ,
ŻĶČ”Fe3+µÄ¹ż³ĢÖŠŅŖæŲÖĘŹŹŅĖµÄĖį¶Č,Čē¹ūČÜŅŗµÄpH¹ż“ó,Ęäŗó¹ūŹĒ”””””””””£
(2)ČēĶ¼ŹĒÓĆĖ«Įņėź(H2Dz)~CCl4ĀēŗĻŻĶČ”Ä³Š©½šŹōĄė×ÓµÄĖį¶ČĒśĻß,Ėü·“Ó³ĮĖŻĶČ”Ä³Š©½šŹōĄė×ÓŹ±ŹŹŅĖµÄpH·¶Ī§”£E%±ķŹ¾Ä³ÖÖ½šŹōĄė×ÓŅŌĀēŗĻĪļŠĪŹ½ŻĶČ”·ÖĄėµÄ°Ł·ÖĀŹ”£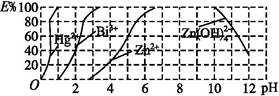
ij¹¤Ņµ·ĻĖ®ÖŠŗ¬ÓŠHg2+”¢Bi3+”¢Zn2+,ÓĆĖ«Įņėź(H2Dz)~CCl4ĀēŗĻŻĶČ”·Ø“¦Ąķ·ĻĖ®”£
¢ŁÓūĶźČ«½«·ĻĖ®ÖŠµÄHg2+·ÖĄė³öĄ“,ŠėæŲÖĘČÜŅŗµÄpH=”””””””””£
¢Śµ±µ÷½ŚpH=2Ź±,īé(Bi)µÄ“ęŌŚŠĪŹ½ÓŠ””””””””,ĘäĪļÖŹµÄĮæÖ®±ČĪŖ”””””””””£
¢ŪŻĶČ”µ½CCl4ÖŠµÄZn(HDz)2·ÖŅŗŗó,¼ÓČė×ćĮæNaOHČÜŅŗ,³ä·ÖÕńµ“ŗó,ŠæÓÖ×Ŗµ½Ė®ČÜŅŗÖŠ”£Š“³ö·“Ó¦µÄĄė×Ó·½³ĢŹ½:
(3)ĪŪĖ®ÖŠµÄŃĒ¹ÆĄė×Ó(H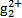 )±ŲŠė×Ŗ»»³É¹ÆĄė×Ó(Hg2+)²ÅÄÜÓĆĖ«ĮņėźĀēŗĻ”£Ä³¹¤³§ĪŪĖ®ÖŠŗ¬ÓŠ½Ļ¶ąµÄĀČ»ÆŃĒ¹Æ(Hg2Cl2),¼ÓČė¶žĮņĖį¼Ų(K2S2O8)æÉŃõ»ÆH
)±ŲŠė×Ŗ»»³É¹ÆĄė×Ó(Hg2+)²ÅÄÜÓĆĖ«ĮņėźĀēŗĻ”£Ä³¹¤³§ĪŪĖ®ÖŠŗ¬ÓŠ½Ļ¶ąµÄĀČ»ÆŃĒ¹Æ(Hg2Cl2),¼ÓČė¶žĮņĖį¼Ų(K2S2O8)æÉŃõ»ÆH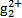 Éś³ÉĮņĖį¹Æ,Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½:”””£
Éś³ÉĮņĖį¹Æ,Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½:”””£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com