
��֪��P4(g)+6Cl2(g)��4PCl3(g) ��H��a kJ∙mol��1 ��P4(g)+10Cl2(g)��4PCl5(g) ��H��b kJ∙mol��1
P4������������ṹ��PCl5��P��Cl���ļ���Ϊc kJ∙mol��1��PCl3��P��Cl���ļ���Ϊ1.2c kJ∙mol��1��
����������ȷ���ǣ� ��
A��P��P���ļ��ܴ���P��Cl���ļ���
B������Cl2(g)+ PCl3(g)��4PCl5(s)�ķ�Ӧ�ȡ�H
C��Cl��Cl���ļ���Ϊ(b��a+5.6c)/4 kJ∙mol��1
D��P��P���ļ���Ϊ(5a��3b+12c)/8 kJ∙mol��1
 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д� �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��98%��Ũ���ᣨ�ܶ�1.84g �� mL-1������100mL 1mol��L-1��ϡ���ᡣ�ָ�����������
�п����õ�����������100 mL��Ͳ ��10 mL��Ͳ ��50 mL�ձ� ��������ƽ
��100 mL����ƿ ��ͷ�ι� �߲���������ʹ���������Ⱥ�˳��������������������
ȷ����
A���ܢۢޢߢݢ� B���ڢޢۢߢݢ� ��
C���٢ۢݢߢݢ� D���ڢۢޢߢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ѹ�������Ͷ�뺬��HCO3-��Cl-��Mg2+��Na+��ˮ��Һ�У�������Ŀ������ǣ� ��
A��HCO3- B��Cl- C��Mg2+ D��Na+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧʵ��С���ͬѧΪ̽���ͱȽ�SO2����ˮ��Ư���ԣ���������µ�ʵ��װ�á�
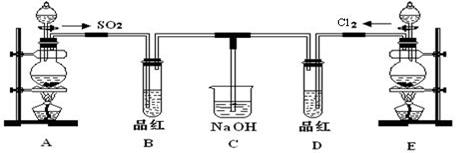
��1��ʵ������װ��A�Ʊ�SO2��ijͬѧ��ʵ��ʱ���ִ�A�ķ�Һ©�������� ©����Һ��δ���£�����Ϊԭ������ǣ�____________________
��2��ʵ������װ��E�Ʊ�Cl2���䷴Ӧ�Ļ�ѧ��ѧ����ʽΪ��MnO2+4HCl��Ũ�� MnCl2+Cl2��+2H2O������6 mol��HCl�μӷ�Ӧ����ת�Ƶĵ��ӵ����ʵ���Ϊ______ mol����������HClΪ mol��
MnCl2+Cl2��+2H2O������6 mol��HCl�μӷ�Ӧ����ת�Ƶĵ��ӵ����ʵ���Ϊ______ mol����������HClΪ mol��
��3���ٷ�Ӧ��ʼһ��ʱ��۲쵽B��D�����Թ��е�Ʒ����Һ���ֵ������ǣ�B��_________________________��D��________________________��
��ֹͣͨ����,�ٸ�B��D�����Թֱܷ���ȣ������Թ��е�����ֱ�Ϊ B��_________________________��D��________________________��
��4����һ��ʵ��С���ͬѧ��ΪSO2����ˮ����Ư���ԣ�����Ϻ��Ư���Կ϶����ǿ�����ǽ��Ƶõ�SO2��Cl2��1��1ͬʱͨ�뵽Ʒ����Һ�У����������ɫЧ���������������������������������ԭ���û�ѧ����ʽ��ʾ�� __________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Գ�����������ƹ�ͨ�磬�ƹܷ�����ɫ�⡣������һ�������Ҫԭ�� ��
A�������ɼ���̬���̬ԾǨʱ�Թ����ʽ�ͷ�����
B�������ɻ�̬��̬ԾǨʱ���ճ��������Ĺ���
C����ԭ�ӻ�õ��Ӻ�ת��ɷ�����������
D���ڵ����������£���ԭ���빹�ɵƹܵ����ʷ�����Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������У���һ�������¼��ܽ��мӳɷ�Ӧ��Ҳ�ܽ���ȡ����Ӧ����������ʹKMnO4������Һ��ɫ����(�� ��)
A������ B����ϩ C���Ҵ� D����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����йؾ���������У��������ǣ� ��
A����SiO2�����У�ÿ��Siԭ����4��Oԭ���γɹ��ۼ�
B�������������ܶѻ��Ľ��������У�ÿ������ԭ����Χ���ڵ���4������ԭ��
C��NaCl��������ÿ��Na+��������������Cl����6����
D��CsCl��������ÿ��Cs+��������������Cl����8��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
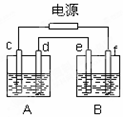
��ͼ��A����ʯī�缫���NaOH��Һ��B�ؾ�����ͭ��һ��ʱ���ֹͣͨ�磬A����d���������������Ե������ڱ�״����Ϊ2.24L������˵����ȷ����
A��A��Ϊ���أ�B��Ϊԭ���
B��������d��e����������������Ӧ
C��B����e��ӦΪ��ͭ�����
D��B����e����������12.8g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(��)���ֺ���Ԫ�ص�����֮����ת����ϵ��ͼ��ʾ��

(ע�⣺����Һ�й۲쵽�������ʵ������H4SiO4��H4SiO4�ڿ�������ʧˮ�γ�H2SiO3)
���жϣ�
(1)д�����м������ʵĻ�ѧʽ��
A________��B________��C________��D________��E________��F________��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ��
��B��A_______________________________________________________________________��
��B��E_______________________________________________________________________��
(3)д��������Һ�з�Ӧ�����ӷ���ʽ��
��A��D_______________________________________________________________________��
��D��F_______________________________________________________________________��
(��)��20 mL 2 mol��L��1 AlCl3��Һ�У�����30 mL NaOH��Һ����ַ�Ӧ��0.78 g������NaOH��Һ�����ʵ���Ũ����________mol��L��1��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com