
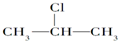 +NaOH$��_{��}^{��}$CH3-CH=CH2+NaCl+H2O
+NaOH$��_{��}^{��}$CH3-CH=CH2+NaCl+H2O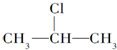 $��_{-NaCl��-H_{2}O}^{NaOH��������}$CH3-CH=CH2
$��_{-NaCl��-H_{2}O}^{NaOH��������}$CH3-CH=CH2
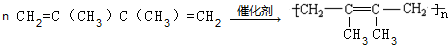 ��
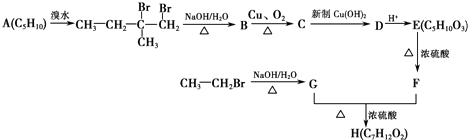
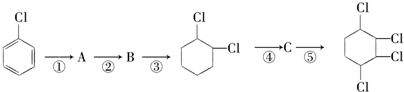
�� ���� A�ڹ�������������������ȡ����Ӧ����B��B���������ƴ���Һ�����������·�����ȥ��Ӧ����C1��C2��C2���巢���ӳɷ�Ӧ����D��D���������ƴ���Һ�����������·�����ȥ��Ӧ����E��E������Է���1��2-�ӳɷ�Ӧ����F2������1��4-�ӳɷ�Ӧ����F1����EΪCH2=C��CH3��C��CH3��=CH2��F2ΪCH2=C��CH3��CBr��CH3��CH2Br��F1ΪBrCH2C��CH3��=C��CH3��CH2Br�����ƿɵ�DΪCH3CBr��CH3��CBr��CH3��2��C2Ϊ��CH3��2C=C��CH3��2��C1Ϊ��CH3��2CHC��CH3��=CH2��
��� �⣺A�ڹ�������������������ȡ����Ӧ����B��B���������ƴ���Һ�����������·�����ȥ��Ӧ����C1��C2��C2���巢���ӳɷ�Ӧ����D��D���������ƴ���Һ�����������·�����ȥ��Ӧ����E��E������Է���1��2-�ӳɷ�Ӧ����F2������1��4-�ӳɷ�Ӧ����F1����EΪCH2=C��CH3��C��CH3��=CH2��F2ΪCH2=C��CH3��CBr��CH3��CH2Br��F1ΪBrCH2C��CH3��=C��CH3��CH2Br�����ƿɵ�DΪCH3CBr��CH3��CBr��CH3��2��C2Ϊ��CH3��2C=C��CH3��2��C1Ϊ��CH3��2CHC��CH3��=CH2��
��1��������A�������ǣ�2��3-�������飬�ʴ�Ϊ��2��3-�������飻
��2��������ͼ�У���Ӧ������ȡ����Ӧ����Ӧ�����ڼӳɷ�Ӧ���ʴ�Ϊ��ȡ����Ӧ���ӳɷ�Ӧ��
��3��D����E�Ļ�ѧ����ʽ��CH3CBr��CH3��CBr��CH3��2+2NaOH$��_{��}^{��}$CH2=C��CH3��C��CH3��=CH2+2NaBr+2H2O��
�ʴ�Ϊ��CH3CBr��CH3��CBr��CH3��2+2NaOH$��_{��}^{��}$CH2=C��CH3��C��CH3��=CH2+2NaBr+2H2O��
��4��C2�Ľṹ��ʽ�ǣ���CH3��2C=C��CH3��2��F1�Ľṹ��ʽ�ǣ�BrCH2C��CH3��=C��CH3��CH2Br��
�ʴ�Ϊ����CH3��2C=C��CH3��2��BrCH2C��CH3��=C��CH3��CH2Br��
��4����E�ϳɸ߷��ӻ�����Ļ�ѧ����ʽ��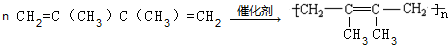 ��
��
�ʴ�Ϊ��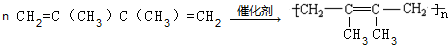 ��
��
���� ���⿼���л�����ƶϣ��Ѷ��еȣ��������չ����ŵ�������ת���ǹؼ�����ѧ������������һ����Ҫ��


 �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
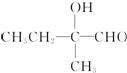 +2Cu��OH��2+OH-$\frac{\underline{\;\;��\;\;}}{\;}$
+2Cu��OH��2+OH-$\frac{\underline{\;\;��\;\;}}{\;}$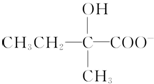 +Cu2O��+3H2O��
+Cu2O��+3H2O��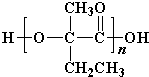 ��
��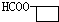 ��
��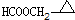 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | KOH | B�� | NaCl | C�� | O2 | D�� | NH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
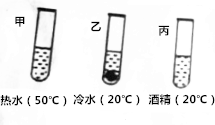 ������Һ�й�֪ʶ���ش��������⣺
������Һ�й�֪ʶ���ش��������⣺| �¶�/�� | 10 | 30 | 50 | 70 | |
| �ܽ��/g | NaCl | 35.8 | 36.3 | 37.0 | 37.8 |
| KNO3 | 20.9 | 45.8 | 85.5 | 138 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
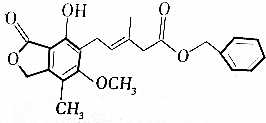
| A�� | �����ʵĻ�ѧʽ��C23H24O6 | |
| B�� | 1mol�û�������������9molH2�ӳ� | |
| C�� | ����KMnO4��Һ����ˮ��������ʷ���������ԭ��Ӧ����ɫ | |
| D�� | ��������FeCl3��Һ���ÿ��Ժ�ɫ���÷�Ӧ�����ڼ������еķ��ǻ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN |
| pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �Ʊ�
�Ʊ�
 B��
B�� ��
�� +2NaOH$��_{��}^{��}$
+2NaOH$��_{��}^{��}$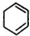 +2NaCl+2H2O
+2NaCl+2H2O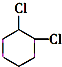 �ĺ�����Ԫ̼����ͬ���칹�壺
�ĺ�����Ԫ̼����ͬ���칹�壺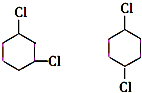
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
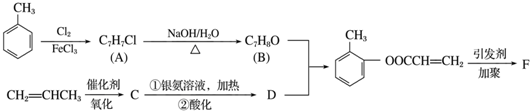
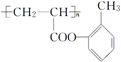 ��
��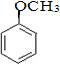 ����ṹ��ʽ����
����ṹ��ʽ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȼú��ȼú������������ | |
| B�� | �ƹ���Ȼ�����״�����Ϊ������ȼ�� | |
| C�� | �Ժ�SO 2��NO 2�ķ������������ſ� | |
| D�� | �˹��ռ�����������ĵ��������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com