
(16��)̼������������������Ҫ�ķǽ���Ԫ�ء�
��1��CH4(g)��O2(g)��ȼ������CO(g)��H2O(g)�ġ�H����ֱ�Ӳ�����ԭ���� ��
��֪��a��2CO(g)+O2(g)=2CO2(g) ��H =-566��0 kJ��mol-1
b��CH4(g)+2O2(g)=CO2(g)+2H2O(g) ��H =-890��0 kJ��mol-1
��CH4(g)��O2(g)��ȼ������CO(g)��H2O(g)���Ȼ�ѧ����ʽΪ ��
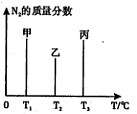
��2����ҵ�Ϻϳɰ����ķ�ӦΪ��N2(g)+3H2(g)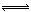 2NH3(g) ��H<0���ֽ�10 mol N2��26 mol H2�����ݻ��ɱ���ܱ������У�N2��ƽ��ת����(
2NH3(g) ��H<0���ֽ�10 mol N2��26 mol H2�����ݻ��ɱ���ܱ������У�N2��ƽ��ת����(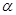 )����ϵ��ѹǿ(P)���¶�(T)�Ĺ�ϵ��ͼ��ʾ���ش��������⣺
)����ϵ��ѹǿ(P)���¶�(T)�Ĺ�ϵ��ͼ��ʾ���ش��������⣺
�ٷ�Ӧ�ﵽƽ��״̬Bʱ���������ݻ�10 L����T1ʱ���ϳɰ���Ӧ��ƽ�ⳣ��K= L2��mol-1��
��ƽ��״̬��A�䵽Cʱ����Ӧ��ƽ�ⳣ��K(A) K(C)(�>������<����=��)��
��3����25��ʱ��HSCN��HClO��H2CO3�ĵ��볣�����±���
| HClO | HSCN | H2CO3 |
| K=3.210-8 | K=0.13 | Kl=4.210-7 K2=5.610-11 |
��1����5�֣����Կ��Ʒ�Ӧֻ����CO(g)��2�֣����ַ�Ӧ���Կ��Ƽ��ɣ�
2CH4(g)+3O2(g)=2CO(g)+4H2O(g) ?H=��1214.0kJ?mol?1��3�֣�
��2����4�֣���0.025��2�֣��� > ��2�֣�
��3����7�֣��� c(K+)>C(SCN?)>c(OH?)>c(H+)��2�֣��������Ʒ֣�
��Na2CO3 + HClO = NaHCO3 +NaClO��3�֣�
��ac��2�֣�
���������������1��CH4ȼ����������CO2�����Կ��Ʒ�Ӧֻ����CO(g)������д��CH4��O2��Ӧ����CO��H2O�Ļ�ѧ����ʽ����ע��״̬��Ȼ����ݸ�˹��������ʱ䣺?H=��?H1+2?H2=��1214.0kJ?mol?1��������д���Ȼ�ѧ����ʽ��
��2���ٷ�Ӧ�ﵽƽ��״̬Bʱ��N2��ת����Ϊ20%����������ʽ���м���
N2(g)+3H2(g)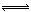 2NH3(g)
2NH3(g)
��ʼ���ʵ�����mol�� 10 26 0
ת�����ʵ�����mol�� 2 6 4
ƽ�����ʵ�����mol�� 8 20 4
��ƽ�ⳣ��Ϊ��K=(0.4mol?L?1)2��[0.8mol/L��(2mol?L?1)3]=0.025 L2��mol-1��
��B��N2ת���ʴ���C�㣬˵��B��ƽ�ⳣ������C��ƽ�ⳣ����A����B���¶���ͬ����ƽ�ⳣ����ͬ������K(A) > K(C)
��3����KSCNΪ����ǿ���Σ�SCN?ˮ��ʹ��Һ�ʼ��ԣ���������Ũ���ɴ�С��˳��Ϊ��c(K+)>C(SCN?)>c(OH?)>c(H+)
����ΪK1(H2CO3)>K(HClO)>K2(H2CO3)������HClO��Na2CO3��Ӧ����NaClO��NaHCO3����ѧ����ʽΪ��Na2CO3 + HClO = NaHCO3 +NaClO
��a����pH�ƿ��Բ���0��1 mol��L-1NaClO��Һ��pH�������pH>7��˵��NaClOˮ���Լ��ԣ���֤��HClOΪ���ᣬ��ȷ��b����ΪHClO����Ư���ԣ�����pH��ֽ����0��01 mol��L-1HClO��Һ��pH������c����ΪHClO������Ũ����ͬ�����������HClO��Һ�ĵ������������ᣬ��֤��HClOΪ���ᣬ��ȷ��
���㣺���⿼���Ȼ�ѧ����ʽ����д��ƽ�ⳣ�����жϺͼ��㡢������ʵĵ��롢�����ˮ�⡢����Ũ�ȱȽϡ�ʵ�鷽���ķ�����


 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д� Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ƽ���ǻ�ѧ��Ӧԭ���е���Ҫ���ݡ���Ҫ��ش��������⣺
��1����֪��2SO2(g)+O2(g) 2SO3(g) ��H1 ƽ�ⳣ��ΪK1
2SO3(g) ��H1 ƽ�ⳣ��ΪK1
2NO(g)+O2(g) 2NO2(g) ��H2 ƽ�ⳣ��ΪK2
2NO2(g) ��H2 ƽ�ⳣ��ΪK2
��ӦNO2(g)+SO2(g) SO3(g)+NO(g)�Ħ�H="______" (�æ�H 1�ͦ�H 2��ʾ)���˷�Ӧ���¶��µ�ƽ�ⳣ��K=______(��K1��K2��ʾ)��
SO3(g)+NO(g)�Ħ�H="______" (�æ�H 1�ͦ�H 2��ʾ)���˷�Ӧ���¶��µ�ƽ�ⳣ��K=______(��K1��K2��ʾ)��
��2����֪A(g)+B(g)  C(g)+D(g)���÷�Ӧ��3L�ܱ������У������ֲ�ͬ�������½��з�Ӧ��A��B����ʼ���ʵ��Ƿֱ�Ϊ3.0mol��6.0mol������ʵ��I������ΪT1�档A�����ʵ�����ʱ��ı仯��ͼ��ʾ��
C(g)+D(g)���÷�Ӧ��3L�ܱ������У������ֲ�ͬ�������½��з�Ӧ��A��B����ʼ���ʵ��Ƿֱ�Ϊ3.0mol��6.0mol������ʵ��I������ΪT1�档A�����ʵ�����ʱ��ı仯��ͼ��ʾ��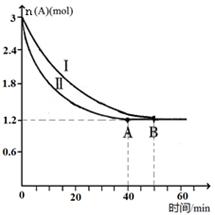
��ʵ�����ܸı��������_______________��
��T1��ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ_____(�÷����� ʾ)���ﵽƽ��ʱ��A�ķ�Ӧ����Ϊ____��
��3����֪HCN��Һ�ĵ���ƽ�ⳣ��Ka=10��5mol?L��1��cƽ��(HCN)��c��ʼ(HCN)��ˮ�ĵ���ɲ��ƣ�����¶���0.1 mol?L��1��HCN��Һ��pH=_________��
��4������0.1mol?L��1 Na2CO3��Һ������Һ������Ũ���ɴ�С��˳����______�������Һ�м�ˮϡ�͵Ĺ����У�c(H2CO3)��____(��������䡱��С���� ��ͬ) ��c(H2CO3)/ c(CO32��)��______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2013���������������Ű�ҹ��ж�����������������β����ȼúβ������ɿ�����Ⱦ��ԭ��֮һ��
��1������β����������Ҫԭ��Ϊ��2NO(g)+2CO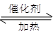 2CO2(g)+N2(g)
2CO2(g)+N2(g)
�ٶ������෴Ӧ����ij��֣�B����ƽ��ѹǿ��PB���������ʵ���Ũ�ȣ�CB��Ҳ���Ա�ʾƽ�ⳣ��������KP������÷�Ӧ��KP=- ��
�ڸ÷�Ӧ�ڵ��������Է����У��÷�Ӧ�Ħ�H 0����ѡ�>������<����
����ijһ���ȡ����ݵ��ܱ������г���һ������NO��CO����������Ӧ���������Ӧ��������ʱ��仯��������ͼ��ʾ����֪��t2 --tl=t3��t2����
������˵������ȷ���� �������ţ�
A����Ӧ��c��δ�ﵽƽ��״̬
B����Ӧ����a��С��b��
C����Ӧ��Ũ��a�����b��
D��NO��ת���ʣ�tl��t2>t2��t3
��2��ú���ۺ����á�ʹ�������Դ�������ڼ��ٻ�����Ⱦ���ϳɰ���ҵԭ��������Դ֮һˮú�������ڴ������������������з�Ӧ��
��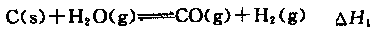
��
��
�١�H3�͡�H1����H2�Ĺ�ϵΪ��H3= ��
���ں��������£���l mol CO��1 mol H2O��g������ij�̶��ݻ��ķ�Ӧ�������ﵽƽ��ʱ��50%��COת��ΪCO2����tlʱ�����¶Ȳ��䣬�ٳ���1 mol H2O��g��������ͼ�л���tlʱ�̺�H2����������仯�������ߡ�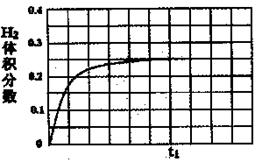
�ۼ״����Ϳɡ��Լ�������β���Ի�������Ⱦ��
ij��������ˮú��Ϊԭ�Ϻϳɼ״������������£�������ɱ���ܱ������з�����Ӧ��CO(g)+2H2(g)  CH3OH(g)����ƽ��ʱ�����CO��H2��CH3OH�ֱ�Ϊ1 mol��1 mol��1 mol�����������Ϊ3L�����������м���ͨ��3 mol CO����ʱv������ v���棩��ѡ���>������<������=�������жϵ����� ��
CH3OH(g)����ƽ��ʱ�����CO��H2��CH3OH�ֱ�Ϊ1 mol��1 mol��1 mol�����������Ϊ3L�����������м���ͨ��3 mol CO����ʱv������ v���棩��ѡ���>������<������=�������жϵ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
11.2 g KOH��ϡ��Һ��1 L 0.1 mol��L-1��H2SO4��Һ��Ӧ�ų�11.46 kJ������,�÷�Ӧ���Ȼ�ѧ����ʽΪ����������������,��KOH��H2SO4���к���Ϊ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪H2(g)��CO(g)��CH3OH(l)��ȼ���ȷֱ�Ϊ285.8 kJ��mol��1��283.0 kJ��mol��1��726.5 kJ��mol��1����ش��������⡣
(1)��̫���ֽܷ�5 molҺ̬ˮ���ĵ������� kJ��
(2)Һ̬�״�����ȫȼ������һ����̼�����Һ̬ˮ���Ȼ�ѧ����ʽΪ ��
(3)���Լ״�Ϊȼ�ϵ�ȼ�ϵ���У��������ҺΪ���ԣ��������ĵ缫��ӦʽΪ ��
����״̬�£���ȼ�ϵ������2 mol�״����ܲ�����������Ϊ1 404.2 kJ�����ȼ�ϵ�ص�����Ч��Ϊ ��(ȼ�ϵ�ص�����Ч����ָ�������������������ȼ�ϵ�ط�Ӧ�����ͷŵ�ȫ������֮��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�ϳ������÷�Ӧ���������������飬���ǰ��渱��Ӧ�ڡ�
��C2H4(g)+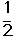 O2(g) ��
O2(g) ��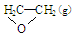 ; ��H1 ��C2H4(g)+3O2(g) �� 2CO2(g)+2H2O(g); ��H2
; ��H1 ��C2H4(g)+3O2(g) �� 2CO2(g)+2H2O(g); ��H2
��1��д������������ȼ�յ��Ȼ�ѧ��Ӧ����ʽ����____________________________��
��2����ҵ�����У���ͨ��ijһ��ʩ���ӿ췴Ӧ�ٶ��Է�Ӧ��Ӱ���С���Ӷ���������������Ч�ʡ���ҵ������ȡ�����ִ�ʩ��_______________��
A�����߷�Ӧ��ϵ���¶�B��������ϵ��������Ũ��
C��ʹ�ú��ʵĴ���D�����ͷ�Ӧ��ϵ��ѹǿ
��3����֪C=C��O=O��C��C���ֱܷ�Ϊa kJ��mol-1��b kJ��mol-1��c kJ��mol-1������������C��O����Ϊ kJ��mol-1��
��4����Ӧ�ڿ�����Ƴ�ȼ�ϵ�أ����������������Һ��������Ӧʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)����ɴ��ϵ�����ȼ�ϵ�أ����ط�ӦΪ��2H2��O2=2H2O����д���������Һ��Ϊ����ʱ��������Ӧʽ��______________��
(2)��֪4 g����������ȼ������CO2(g)��H2O(l)ʱ���ų�Q kJ��������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��______________________��
(3)��֪�����Ȼ�ѧ����ʽ��
��Fe2O3(s)��3CO(g)=2Fe(s)��3CO2(g)����H����Q1 kJ/mol
��3Fe2O3(s)��CO(g)=2Fe3O4(s)��CO2(g)����H����Q2 kJ/mol
��Fe3O4(s)��CO(g)=3FeO(s)��CO2(g)����H����Q3 kJ/mol
���ø�˹���ɼ��㣺FeO(s)��CO(g)=Fe(s)��CO2(g)���ʱ䦤H��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ܡ�������δ�������������Դ֮һ��
��1��ʵ���ã�1 g����ȼ������Һ̬ˮʱ�ų�142.9 kJ���������ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ__________________________��
��2�����ú��ܰ�ˮ�ֽ�����������Ŀǰ���о��Ŀ��⡣��ͼ�����е�һ�����̣����������˹����ĵ⣨��ʾ����Ӧ�ڵIJ�����O2��SO2��H2O����
������з�Ӧ�Ļ�ѧ����ʽ����Ӧ��________________����Ӧ��________________���˷���ȡ����������ŵ���____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���á�
��1���ڹ̶��ݻ����ܱ������У��������»�ѧ��Ӧ��N2��g��+3H2��g�� 2NH3��g�� ��H=��92��4kJ/mol��
2NH3��g�� ��H=��92��4kJ/mol��
��ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
| T/K | 298 | 398 | 498 |
| ƽ�ⳣ��K | 4.1��106 | K1 | K2 |
 ��NH2��2CO +H2O
��NH2��2CO +H2O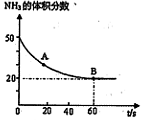
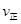 ��CO2�� B����淴Ӧ����
��CO2�� B����淴Ӧ����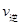 ��CO2������д��>����=����<������NH3��ƽ��ת����Ϊ____ ��
��CO2������д��>����=����<������NH3��ƽ��ת����Ϊ____ �� 2NO��g�� ��H =+180kJ/mol
2NO��g�� ��H =+180kJ/mol�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com