
(8��)��ͼ��ʾ����һ���㡢Ϻ���������ĺӱߣ������ε����������мס��ҡ����������������������ŷŵķ�ˮ�ﶼֻ����Na2CO3��FeCl3��Ca(OH)2��HCl�е�һ�֡�ij��ѧ����С��Ժ�ˮ���ʱ���֣��ټ״���ˮ�����ɫ�����Ҵ���ˮ�ʺ��ɫ���۱�����ˮ�ɻ���壻�ܶ����������ݣ���ˮ�Գ��塣��ش�
��1�����������ų��ķ�ˮ�ﺬ�е���Ⱦ��ֱ�Ϊ��
�ף� �ң� ���� ���� ���ѧʽ��
��2���ڶ�������M��ȡ���ĺ�ˮ�У��϶����е������� ��
��3��д���йط�Ӧ�����ӷ���ʽ��
��
 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014�������ʡ�߶���ѧ���ڳ����Ի�ѧ�Ծ��������棩 ���ͣ������
(8��)��A��B��C��D���ֶ�����Ԫ�أ���ԭ��������������A��B���γ�A2B��A2B2���ֻ����B��Cͬ�����ҿ��γ�CB2��CB3���ֻ�����ش��������⡣
(1)A2B2�ĵ���ʽΪ____________��
(2)CB2ͨ��A2B2��Һ�пɱ�����ΪW����W����Һ(���Ϊ1 L������仯ǰ����Һ����仯���Բ���)��װ��ԭ���(��ͼ��ʾ)��
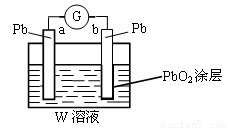
��b�缫�Ϸ����ķ�Ӧ�ɱ�ʾΪ��PbO2��4H����SO ��2e����PbSO4��2H2O������a�缫�Ϸ����ķ�Ӧ�ɱ�ʾΪ_________________������ع���һ��ʱ���a������0.05 mol Pb����W��Ũ������������39 % (�ܶ�1.3 g / cm3)��Ϊ______mol
/L��
��2e����PbSO4��2H2O������a�缫�Ϸ����ķ�Ӧ�ɱ�ʾΪ_________________������ع���һ��ʱ���a������0.05 mol Pb����W��Ũ������������39 % (�ܶ�1.3 g / cm3)��Ϊ______mol
/L��
(3)����Ԫ��E����ѧ��ѧ����Ԫ�أ�λ��Ԫ�����ڱ��ĵ������ڡ���Ԫ�ؿ���D�γ�ED2��ED3���ֻ������E�ĵ��ʽ���ED3��Һ��(����ͼ����ʾ)����Һ�ɻ�ɫ��Ϊdz��ɫ���÷�Ӧ�����ӷ���ʽΪ____________________________________��

(4)����(3)�еķ�Ӧ�����õ���E��ʯīΪ�缫���һ��ԭ��أ�ͼ�ң������ڸ�ԭ��ع���ʱ��ʯīһ�������ķ�Ӧ���Ա�ʾΪ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡͩ���и�����ѧ��10�·��¿���ѧ�Ծ� ���ͣ�ʵ����
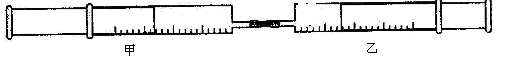
������8�֣���ͼ��ʾ�����ס�������װ�в�ͬ���ʵ���Ͳ�õ�������������������Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ���ʵ�飨������ͬ��ͬѹ�²ⶨ����
�Իش��������⣺

��1��ʵ��1�У��������ձ�Ϊ�� ɫ��д��������ɫ�Ļ�ѧ����ʽ ��
��2��ʵ��2�У���֪��3Cl2+2NH3=N2+6HCl������Ͳ���������ƶ�, ��Ͳ���а��̲����⣬�������ɫ�仯Ϊ �����������Ͳ�й�ʣ����������ԼΪ mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������8�֣���ͼ��ʾ�����ס�������װ�в�ͬ���ʵ���Ͳ�õ�������������������Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ���ʵ�飨������ͬ��ͬѹ�²ⶨ����
�Իش��������⣺
��1��ʵ��1�У��������ձ�Ϊ�� ɫ��д��������ɫ�Ļ�ѧ����ʽ ��
��2��ʵ��2�У���֪��3Cl2+2NH3=N2+6HCl������Ͳ���������ƶ�, ��Ͳ���а��̲����⣬�������ɫ�仯Ϊ �����������Ͳ�й�ʣ����������ԼΪ mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡͩ���и���ѧ������ѧ��10�·��¿���ѧ�Ծ� ���ͣ�ʵ����
������8�֣���ͼ��ʾ�����ס�������װ�в�ͬ���ʵ���Ͳ�õ����������� ��������Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ���ʵ�飨������ͬ��ͬѹ�²ⶨ����
��������Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ���ʵ�飨������ͬ��ͬѹ�²ⶨ����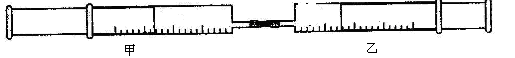
�Իش��������⣺
��1��ʵ��1�У��������ձ�Ϊ�� ɫ��д��������ɫ �Ļ�ѧ����ʽ ��
�Ļ�ѧ����ʽ ��
��2��ʵ��2�У���֪��3Cl2+2NH3=N2+6HCl������Ͳ���������ƶ�, ��Ͳ���а��̲����⣬�������ɫ�仯Ϊ �����������Ͳ�й�ʣ����������ԼΪ mL��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com