
����Ŀ��I.д�����з�Ӧ���Ȼ�ѧ����ʽ��
��1��CuCl(s)��O2��Ӧ����CuCl2(s)��һ�ֺ�ɫ���塣��25 ����101 kPa�£���֪�÷�Ӧÿ����1 mol CuCl(s)������44.4 kJ���÷�Ӧ���Ȼ�ѧ����ʽ��_____________________��
��2����1.01��105 Paʱ��16 g S�����������������г��ȼ�����ɶ������ų�148.5 kJ����������S����ȼ���ȵ��Ȼ�ѧ����ʽΪ________________________��
II.�о�NOx��SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���塣
��3��������CO��SO2�̵�����Ⱦ��һ�ַ����ǽ����ڴ���������ת��Ϊ����S���塣��֪��
��CO(g)��![]() O2(g)=CO2(g) ��H����283.0 kJ��mol��1
O2(g)=CO2(g) ��H����283.0 kJ��mol��1
��S(s)��O2(g)=SO2(g)�� ��H����296.0 kJ��mol��1
�˷�Ӧ���Ȼ�ѧ����ʽ��_____________________��
��4��������������ɹ⻯ѧ�����ͳ�������ĵ���Ҫ���塣��֪��
CO(g)��NO2(g)=NO(g)��CO2(g) ��H����a kJ��mol��1(a>0)
2CO(g)��2NO(g)=N2(g)��2CO2(g) ��H����b kJ��mol��1(b>0)
���ñ�״����3.36 L CO��ԭNO2��N2(CO��ȫ��Ӧ)������������ת�Ƶ��ӵ����ʵ���Ϊ________mol���ų�������Ϊ______________kJ(�ú���a��b�Ĵ���ʽ��ʾ)��
���𰸡�4CuCl(s)��O2(g)=2CuCl2(s)��2CuO(s) ��H����177.6 kJ��mol��1 S(s)+O2(g)SO2(g)����H=-297 kJ��mol-1 2CO(g)��SO2(g)= S(s)��2CO2(g) ��H����270 kJ��mol��1 0.3 ![]()
��������
I.��1����ɫ����Ϊ����ͭ������1 mol CuCl(s)������44.4 kJ������4 mol CuCl(s)������177.6 kJ���ݴ���д�Ȼ�ѧ����ʽ��
��2��16 g ����0.5mol��S�����������������г��ȼ�����ɶ���������148.5 kJ�� 1molS�����������������г��ȼ�����ɶ���������297 kJ���ݴ���д�Ȼ�ѧ����ʽ��
II.��3����4�����ø�˹���ɽ��м��㣬����д��ȷ���Ȼ�ѧ����ʽ��
I.��1����ɫ����Ϊ����ͭ������1 mol CuCl(s)������44.4 kJ������4 mol CuCl(s)������177.6 kJ���Ȼ�ѧ����ʽΪ��4CuCl(s)��O2(g)=2CuCl2(s)��2CuO(s) ��H����177.6 kJ��mol��1��
��2��16 g ����0.5mol��S�����������������г��ȼ�����ɶ���������148.5 kJ�� 1molS�����������������г��ȼ�����ɶ���������297 kJ���Ȼ�ѧ����ʽΪ��S(s)+O2(g)SO2(g)����H=-297 kJ��mol-1��
II.��3�����ݸ�˹������2����-�ڵ��Ȼ�ѧ����ʽΪ��2CO(g)��SO2(g)= S(s)��2CO2(g) ��H����270 kJ��mol��1��
��4����֪����CO(g)��NO2(g)=NO(g)��CO2(g) ��H����a kJ��mol��1(a>0)
��2CO(g)��2NO(g)=N2(g)��2CO2(g) ��H����b kJ��mol��1(b>0)
���ݸ�˹������2����+�ڵã�4CO(g)��2NO2(g)= N2(g)��4CO2(g) ��H����(2a +b��kJ��mol��1��4molCO ��ԭNO2��N2������ת��8mol���ų�������Ϊ(2a +b��kJ���ñ�״����3.36 L(��0.15mol) CO��ԭNO2��N2������ת��0.3mol���ų�������Ϊ![]() kJ��
kJ��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ķ�Ӧ·��������Ϣ��ա�
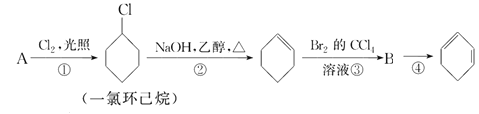
(1)A�Ľṹ��ʽ��________��������________��
(2)�ٵķ�Ӧ������________���۵ķ�Ӧ������________��
(3)��Ӧ�ڵĻ�ѧ����ʽ��_____________________________��
(4)��Ӧ�۵Ļ�ѧ����ʽ��_____________________________��
(5)��Ӧ�ܵĻ�ѧ����ʽ��_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��1892�����ʱ������ά��NaCl��CO2��NH3��H2OΪԭ���Ƶ��˴�����Na2CO3���÷��ֳư��������Ҫ�����������¡�����������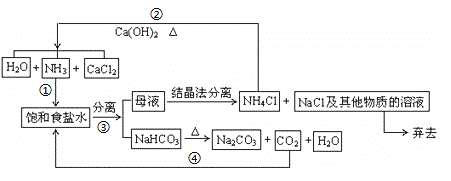
(1) д����Ӧ���в���̼�����Ƶ����ӷ���ʽ_________________________________��
(2)NaHCO3��ˮ���ܽ�Ƚ�С���Գ�����ʽ��������ͼ�Т۵IJ�������___________________������ɫ��ѧԭ�ϵij�����õĽǶȿ����÷��������Ե�ȱ�ݣ���һ�����ɣ�___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�����������Ļ�ѧʽ�ɱ�ʾΪKa[Feb(C2O4)c]��xH2O��Ϊ�ⶨ����ɣ���������ʵ�飺
����1����ȡ1.9640g������ᄃ�壬���Ƴ�250.00mL��Һ��
����2��ȡ������Һ25.00mL����ƿ�У�����1mol��L-1����5.0mL�����ȵ�70~85������0.0100mol��L-1KMnO4��Һ�ζ����յ㣬����KMnO4��Һ48.00mL��
����3����Ӧ�����Һ�м���һ����п�ۡ���������ɫǡ����ʧ�����ˣ�ϴ�ӣ������˼�ϴ��������Һ�ռ�����ƿ�У���ʱ��Һ�Գ����ԡ�
����4��������0.0100mol��L-1KMnO4��Һ�ζ�����3������Һ���յ㣬����KMnO4��Һ8.00mL��
(1)����2�У�KMnO4��C2O![]() ����ΪCO2���õζ���Ӧ�����ӷ���ʽΪ___��
����ΪCO2���õζ���Ӧ�����ӷ���ʽΪ___��
(2)����3�л�ɫ��ʧ��ԭ����___(�����ӷ���ʽ��ʾ)��
(3)�����������Һ�Ĺ����У�������ʱ��������ƿ�Ŀ̶��ߣ����������þ��������ˮ�ĺ���___(����ƫ������ƫС��������Ӱ����)��
(4)ͨ������ȷ���������������Ļ�ѧʽ___(д���������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л��Ļ�ԴԶ��������![]() ����ͼ��
����ͼ��![]() �����ж��෯������������������
�����ж��෯������������������![]() ����ɫ��ȡ�������������ϣ���̼����֮�Ҵ��������㣬�䷯���У�������ɫ�����ڽ���������Ҳ����������������������˵����ȷ����
����ɫ��ȡ�������������ϣ���̼����֮�Ҵ��������㣬�䷯���У�������ɫ�����ڽ���������Ҳ����������������������˵����ȷ����![]() ����
����![]()
A.�����Ȳ����ڵ����Ҳ�����ڷǵ����
B.�෯�ijɷ�Ϊ![]()
C.����ʹ���������ᾧ�ķ��뷽��
D.���еķ�Ӧ�漰��������ԭ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I. ��50 mL 0.50 mol��L��1������50 mL 0.55 mol��L��1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺

��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ���������________��
��2�������60 mL 0.50 mol��L��1������50 mL 0.55 mol��L��1NaOH��Һ���з�Ӧ��������ʵ����ȣ������к���________(��������������������)��
��3��ʵ��ʱ�������ἰNaOH��Һ�������Ϊ50 mL������Һ�ܶȾ�Ϊ1 g��mL��1��������Һ�ı�����c��4.18 J��g��1������1��ʵ����ʼ�¶�Ϊt1������ֹ�¶�Ϊt2�������ƶ��к��ȵļ���ʽ��H��________��
��4������ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��________(����ƫ��������ƫС��������Ӱ����)��
II.ij�о���ѧϰС������H2C2O4��Һ������KMnO4��Һ�ķ�Ӧ̽������������ĸı�Ի�ѧ��Ӧ���ʵ�Ӱ����������������ʵ�飺
ʵ����� | ʵ���¶�/K | �й����� | ��Һ��ɫ������ɫ����ʱ��/s | ||||
����KMnO4��Һ | H2C2O4��Һ | H2O | |||||
V/mL | c/ mol��L��1 | VmL | c/ mol��L��1 | V/mL | |||
A | 293 | 2 | 0.02 | 4 | 0.1 | 0 | t1 |
B | T1 | 2 | 0.02 | 3 | 0.1 | V1 | 8 |
C | 313 | 2 | 0.02 | V2 | 0.1 | 1 | t2 |
��1��ͨ��ʵ��A��B����̽����________(���ⲿ����)�ĸı�Ի�ѧ��Ӧ���ʵ�Ӱ�죬����V1��________��T1��________��ͨ��ʵ��________(��ʵ�����)��̽�����¶ȱ仯�Ի�ѧ��Ӧ���ʵ�Ӱ�죬����V2��________��
��2����t1��8�����ɴ�ʵ����Եó��Ľ�����________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����![]() ����ͬ���壬��Ԫ�ؼ��仯���������彡������ҵ����������ء�ij����С����������
����ͬ���壬��Ԫ�ؼ��仯���������彡������ҵ����������ء�ij����С����������![]() ��Ҫ�ɷ���Se������CuSe��
��Ҫ�ɷ���Se������CuSe��![]() ������
������![]() Ϊԭ�ϣ����������������£�
Ϊԭ�ϣ����������������£�
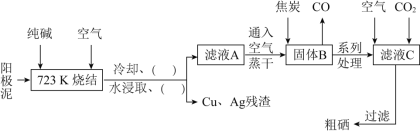
��ش��������⣺
![]() ��ԭ�ӵĴ���������Ϊ________������ͬ��������Ԫ����________
��ԭ�ӵĴ���������Ϊ________������ͬ��������Ԫ����________![]() ��Ԫ������
��Ԫ������![]() ��
��
![]() ��֪��ҺA����Ҫ�ɷ���
��֪��ҺA����Ҫ�ɷ���![]() �����������ƿ�ɽ������
�����������ƿ�ɽ������![]() �Ļ�ѧ����Ϊ________����ҺC����Ҫ�ɷ���
�Ļ�ѧ����Ϊ________����ҺC����Ҫ�ɷ���![]() ����
����![]() �ĵ���ʽΪ________��
�ĵ���ʽΪ________��
![]() ��������ͼ�е���������
��������ͼ�е���������![]()
![]() �����������Ⱥ�˳��������д��������________��________��
�����������Ⱥ�˳��������д��������________��________��
![]() д���������ý�̿��ԭ����B�Ļ�ѧ����ʽ________��
д���������ý�̿��ԭ����B�Ļ�ѧ����ʽ________��
![]() ��ҺC�������������ӷ���ʽΪ________��
��ҺC�������������ӷ���ʽΪ________��
![]() ��
��![]() ��Һ�еμ��Թ��������ᣬ�����ӷ���ʽΪ________����֪��
��Һ�еμ��Թ��������ᣬ�����ӷ���ʽΪ________����֪��![]() ��
��![]() ��
��![]() ��
��
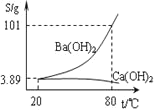
![]() �����ɲ����������ķ��������ᴿ����ô������������Ļӷ��������������¶ȵĹ�ϵ��ͼ��ʾ��
�����ɲ����������ķ��������ᴿ����ô������������Ļӷ��������������¶ȵĹ�ϵ��ͼ��ʾ��

��������п��Ƶ�����¶���________![]() ����
����![]() ��
��
A ![]() B
B ![]() C
C ![]() D
D ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼�ᱵ��һ����Ҫ�����β�Ʒ���㷺Ӧ���ڹ�ҵ�մɡ���ѧ��������������ҵ��
![]() �����Ʊ�����ҵ��һ�����ؾ�ʯ
�����Ʊ�����ҵ��һ�����ؾ�ʯ![]() Ϊԭ�ϣ����ø������ջ�ԭ����ʵ����һ�����ؾ�ʯ
Ϊԭ�ϣ����ø������ջ�ԭ����ʵ����һ�����ؾ�ʯ![]() Ϊԭ�ϣ����ó���ת������
Ϊԭ�ϣ����ó���ת������
�������ջ�ԭ����
![]()
![]() ���ջ�ԭ�Ļ�ѧ����ʽΪ��_____________��
���ջ�ԭ�Ļ�ѧ����ʽΪ��_____________��
![]() Ϊ����߽�ȡ���ʣ��ɲ�ȡ�Ĵ�ʩ��__________
Ϊ����߽�ȡ���ʣ��ɲ�ȡ�Ĵ�ʩ��__________![]() ��дһ��
��дһ��![]() ��
��
![]() ������BaS��ˮ��Һ�ʼ��ԣ�ԭ����
������BaS��ˮ��Һ�ʼ��ԣ�ԭ����![]() �����ӷ���ʽ��ʾ
�����ӷ���ʽ��ʾ![]() __________��
__________��
����ת��������![]() �����м��뱥��
�����м��뱥��![]() ��Һ����ֽ��裬��ȥ�ϲ���Һ����˴�����Σ�ֱ��
��Һ����ֽ��裬��ȥ�ϲ���Һ����˴�����Σ�ֱ��![]() ȫ��ת��Ϊ
ȫ��ת��Ϊ![]() ��
��![]() ƽ�ⳣ��
ƽ�ⳣ��![]()
![]() ����
����![]() ��ÿ����
��ÿ����![]() ����
����![]() ��Һ�������ٶ�
��Һ�������ٶ�![]() ��ȫ��ת����������Ҫ����_____________�Ρ�
��ȫ��ת����������Ҫ����_____________�Ρ�
![]() ������ɫ��ѧ���Ƕȷ������ñ���
������ɫ��ѧ���Ƕȷ������ñ���![]() ��Һ����ת�������ŵ��ǣ�__________��
��Һ����ת�������ŵ��ǣ�__________��
![]() �����ᴿ
�����ᴿ
ijʵ���ҷ����ᴿ��������̼������ʵ�̼�ᱵ��Ʒ�IJ������£�
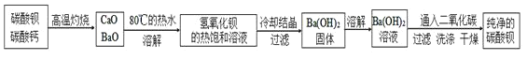
![]() �����պ�Ĺ�����������
�����պ�Ĺ�����������![]() ����ˮ�У��Ƴ������������ȱ�����Һ��Ϊ����
����ˮ�У��Ƴ������������ȱ�����Һ��Ϊ����![]() ����ʧ����ȥ������Ӧ���еIJ�����_____��
����ʧ����ȥ������Ӧ���еIJ�����_____��
![]() ���Ƶõ�
���Ƶõ�![]() ��Һ�еμ�_______
��Һ�еμ�_______![]() ��һ���Լ�����
��һ���Լ�����![]() ����ͨ�������̼�����۲쵽_______ʱ������ֹͣͨ������̼��
����ͨ�������̼�����۲쵽_______ʱ������ֹͣͨ������̼��
![]() �������
�������
![]() �������ij������ˮ��
�������ij������ˮ��![]() ��Ũ�ȡ�ȡ��ˮ
��Ũ�ȡ�ȡ��ˮ![]() �������ʵ�����ȼ���������
�������ʵ�����ȼ���������![]() ��Һ����
��Һ����![]() ������������ϴ�ӡ����˺���������ϡ�����ܽ⣬��ʱ
������������ϴ�ӡ����˺���������ϡ�����ܽ⣬��ʱ![]() ȫ��ת��Ϊ
ȫ��ת��Ϊ![]() ���ټ������KI��Һ����ӦҺ���ٵμ�
���ټ������KI��Һ����ӦҺ���ٵμ�![]() ��Һ����Ӧ��ȫʱ������
��Һ����Ӧ��ȫʱ������![]() ��Һ
��Һ![]() ����֪�йص����ӷ���ʽΪ��
����֪�йص����ӷ���ʽΪ��![]() ��
��![]() ��
��
�ù�����ˮ��![]() �����ʵ���Ũ��__________
�����ʵ���Ũ��__________![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Se�����仯�����ڹ�ũҵ��������������;����ͭ�����ࣨ��Ҫ�ɷ�ΪAg2Se��Cu2Se�������𡢲��ȣ�Ϊԭ���Ʊ������Ĺ���������ͼ��ʾ��
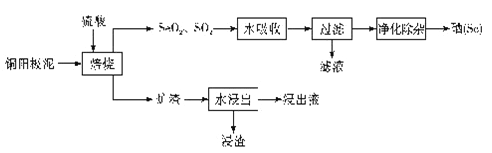
�ش��������⣺
��1����������ʱ������Ũ�����Ϊ_____�����ţ���
A��Ũ���� B�� 20������ C��50������ D�� 80%����
��2����������������Cu2Se���뷴Ӧʱ���÷�Ӧ������������_______��
��3����ˮ��������������Ӧ�Ļ�ѧ����ʽΪ____________��
��4�������������ô����ɲ����������ķ��������ᴿ����ô������������Ļӷ��������������¶ȵĹ�ϵ��ͼ��ʾ��
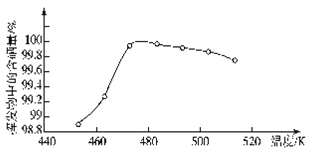
��������п��Ƶ�����¶���_____�����ţ���
A��455�� B��462�� C��475�� D��515��
��5����ˮ�������е�¯������飬�Ҽ�����ˮ���н��ݣ�Ŀ����____________________�����������к��еĽ���������___________��
��6����������Һ���У�c(Ag+)=3.0��10-2 mol/L������Һ��c(SO42-)���Ϊ___________________����֪��Ksp(Ag2SO4)=1.4��10-5������������2λ��Ч���֣���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com