
³£ĪĀĻĀ£¬Ļņ100 mL 0.01 mol”¤L£1 HAČÜŅŗÖŠÖšµĪ¼ÓČė0.02 mol”¤L£1 MOHČÜŅŗ£¬Ķ¼ÖŠĖłŹ¾ĒśĻß±ķŹ¾»ģŗĻČÜŅŗµÄpH±ä»ÆĒéæö(Ģå»ż±ä»ÆŗöĀŌ²»¼Ę)”£»Ų“šĻĀĮŠĪŹĢā£ŗ

(1)ÓÉĶ¼ÖŠŠÅĻ¢æÉÖŖHAĪŖ________Ėį(Ģī”°Ēæ”±»ņ”°Čõ”±)£¬ĄķÓÉŹĒ
__________________________________________________________ӣ
(2)³£ĪĀĻĀŅ»¶ØÅØ¶ČµÄMAĻ”ČÜŅŗµÄpH£½a£¬Ōņa________7(Ģī”°£¾”±”¢”°£¼”±»ņ”°£½”±)£¬ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾ĘäŌŅņ£ŗ________________£¬“ĖŹ±£¬ČÜŅŗÖŠÓÉĖ®µēĄė³öµÄc(OH£)£½____________”£
(3)ĒėŠ“³öKµćĖł¶ŌÓ¦µÄČÜŅŗÖŠĄė×ÓÅØ¶ČµÄ“óŠ”¹ŲĻµ£ŗ________________________________”£
(4)Kµć¶ŌÓ¦µÄČÜŅŗÖŠ£¬c(M£«)£«c(MOH)________2c(A£)(Ģī”°£¾”±”¢”°£¼”±»ņ”°£½”±)£»Čō“ĖŹ±ČÜŅŗµÄpH£½10£¬Ōņc(MOH)£«c(OH£)”Ö________mol”¤L£1”£
½āĪö””(1)ÓÉĢāÄæŠÅĻ¢æÉÖŖ0.01 mol”¤L£1 HAČÜŅŗµÄpH£½2£¬ĖµĆ÷ĘäĶźČ«µēĄė£¬¹ŹĪŖĒæµē½āÖŹ”£(2)ÓÉĢāÄæĶ¼ĻńæÉÖŖĻņ100 mL 0.01 mol”¤L£1 HAČÜŅŗÖŠµĪ¼Ó51 mL 0.02 mol”¤L£1 MOHČÜŅŗ£¬pH£½7£¬ĖµĆ÷MOHŹĒČõ¼ī£¬¹ŹĘäĖł¶ŌÓ¦µÄMAŹĒĒæĖįČõ¼īŃĪ£¬Ė®½āĻŌĖįŠŌ£¬ČÜŅŗÖŠµÄH£«Č«²æŹĒĖ®µēĄė³öĄ“µÄ£¬¹ŹĖ®µēĄė³öµÄc(OH£)£½1”Į10£a mol”¤L£1”£(3)KµćŹĒÓÉ100 mL 0.01 mol”¤L£1 HAČÜŅŗÓė100 mL 0.02 mol”¤L£1 MOHČÜŅŗ»ģŗĻ¶ų³ÉµÄ£¬·“Ó¦ŗóµÄČÜŅŗĪŖµČĪļÖŹµÄĮæÅØ¶ČµÄMAŗĶMOHČÜŅŗ£¬¹Źc(M£«)£¾c(A£)£¾c(OH£)£¾c(H£«)”£(4)ÓÉĪļĮĻŹŲŗćµĆc(M£«)£«c(MOH)£½2c(A£)£¬ÓɵēŗÉŹŲŗćµĆc(M£«)£«c(H£«)£½c(A£)£«c(OH£)£¬¹Źc(MOH)£«c(OH£)£½c(A£)£«c(H£«)”Ö0.005 mol”¤L£1”£
“š°ø””(1)Ēæ””0.01 mol HAČÜŅŗÖŠc(H£«)£½0.01 mol”¤L£1£¬ĘäpHĪŖ2
(2)£¼””M£«£«H2OMOH£«H£«””1”Į10£a mol”¤L£1
(3)c(M£«)£¾c(A£)£¾c(OH£)£¾c(H£«)
(4)£½””0.005



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ö±½ÓÅÅ·Åŗ¬SO2µÄŃĢĘų»įŠĪ³ÉĖįÓź£¬Ī£ŗ¦»·¾³”£ĄūÓĆÄĘ¼īŃ»··ØæÉĶŃ³żŃĢĘųÖŠµÄSO2”£
(1)ĪüŹÕŅŗĪüŹÕSO2µÄ¹ż³ĢÖŠ£¬pHĖęn(SO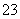 )”Ćn(HSO
)”Ćn(HSO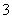 )±ä»Æ¹ŲĻµČēĻĀ±ķ£ŗ
)±ä»Æ¹ŲĻµČēĻĀ±ķ£ŗ
| n(SO | 91”Ć9 | 1”Ć1 | 9”Ć91 |
| pH | 8.2 | 7.2 | 6.2 |
ÓÉÉĻ±ķÅŠ¶Ļ£¬NaHSO3ČÜŅŗĻŌ________ŠŌ£¬ÓĆ»ÆŃ§Ę½ŗāŌĄķ½āŹĶ£ŗ________________________________________________________________________”£
(2)µ±ĪüŹÕŅŗµÄpH½µÖĮŌ¼ĪŖ6Ź±£¬ŠčĖĶµ½µē½ā²ŪŌŁÉś”£ŌŁÉśŹ¾ŅāĶ¼ČēĻĀ£ŗ
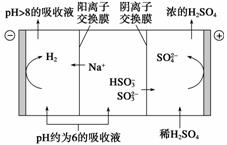
µ±Ņõ¼«ŹŅÖŠČÜŅŗpHÉżÖĮ8ŅŌÉĻŹ±£¬ĪüŹÕŅŗŌŁÉś²¢Ń»·ĄūÓĆ”£¼ņŹöŌŁÉśŌĄķ£ŗ
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
»ÆѧÓėÉē»į”¢Éś²ś”¢Éś»īĆÜĒŠĻą¹Ų”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
A£®ŹÆÓ¢Ö»ÄÜÓĆÓŚÉś²ś¹āµ¼ĻĖĪ¬
B£®“Óŗ£Ė®ÖŠĢįČ”ĪļÖŹ¶¼±ŲŠėĶعż»Æѧ·“Ó¦²ÅÄÜŹµĻÖ
C£®ĪŖĮĖŌö¼ÓŹ³ĪļµÄÓŖŃų³É·Ö£¬æÉŅŌ“óĮæŹ¹ÓĆŹ³Ę·Ģķ¼Ó¼Į
D£®”°µŲ¹µÓĶ”±½ūÖ¹Ź³ÓĆ£¬µ«æÉŅŌÓĆĄ“ÖĘ·ŹŌķ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
25”ꏱ£¬ĻÖÓŠ0.1 mol”¤L£1µÄ°±Ė®”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ĪŖĮĖÖ¤Ć÷Ņ»Ė®ŗĻ°±(NH3”¤H2O)ŹĒČõµē½āÖŹ£¬³£ĪĀĻĀ£¬¼×”¢ŅŅ”¢±ūČżČĖ·Ö±šŃ”ÓĆĻĀĮŠŹŌ¼Į½ųŠŠŹµŃé£ŗ0.010 mol”¤L£1°±Ė®”¢0.1 mol”¤L£1 NH4ClČÜŅŗ£¬NH4Cl¾§Ģ唢·ÓĢŖŹŌ¼Į”¢pHŹŌÖ½”¢ÕōĮóĖ®”£
¢Ł¼×ÓĆpHŹŌÖ½²ā³ö0.010 mol”¤L£1°±Ė®µÄpHĪŖ10£¬ŌņČĻ¶ØŅ»Ė®ŗĻ°±ŹĒČõµē½āÖŹ£¬ÄćČĻĪŖÕāŅ»·½·ØŹĒ·ńÕżČ·£æ________(Ģī”°ŹĒ”±»ņ”°·ń”±)£¬ĒėĖµĆ÷ĄķÓÉ£ŗ__________________________________________________________
__________________________________________________________ӣ
¢ŚŅŅČ”³ö10 mL 0.010 mol”¤L£1°±Ė®£¬ÓĆpHŹŌÖ½²ā³öĘäpH£½a£¬Č»ŗóÓĆÕōĮóĖ®Ļ”ŹĶÖĮ1 000 mL£¬ŌŁÓĆpHŹŌÖ½²ā³öpHĪŖb£¬ČōŅŖČ·ČĻNH3”¤H2OŹĒČõµē½āÖŹ£¬Ōņa”¢bÓ¦Āś×ć¹ŲĻµ£ŗ________________(ÓƵȏ½»ņ²»µČŹ½±ķŹ¾)”£
¢Ū±ūČ”³ö10 mL 0.010 mol”¤L£1°±Ė®£¬µĪČė2µĪ·ÓĢŖŹŌ¼Į£¬ĻŌ·ŪŗģÉ«£¬ŌŁ¼ÓČėÉŁĮæNH4Cl¾§Ģ壬ŃÕÉ«±ä________(Ģī”°Éī”±»ņ”°Ē³”±)”£ÄćČĻĪŖÕāŅ»·½·ØÄÜ·ńÖ¤Ć÷NH3”¤H2OŹĒČõµē½āÖŹ£æ________(Ģī”°ÄÜ”±»ņ”°·ń”±)£¬²¢ĖµĆ÷ŌŅņ£ŗ__________________________________________________________
__________________________________________________________ӣ
(2)ČōĻņ°±Ė®ÖŠ¼ÓČėÉŁĮæĮņĖįļ§¹ĢĢ壬“ĖŹ±ČÜŅŗÖŠ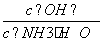 ________(Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±)”£
________(Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±)”£
(3)ČōĻņ°±Ė®ÖŠ¼ÓČėĻ”ĮņĖį£¬¶žÕßĒ”ŗĆĶźČ«·“Ó¦ŗóĖłµĆČÜŅŗµÄpH________7(Ģī”°£¾”±”¢”°£¼”±»ņ”°£½”±)£¬ÓĆĄė×Ó·½³ĢŹ½½āŹĶŌŅņ£ŗ__________________________________________________________
__________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
25 ”ꏱ£¬ĻĀĮŠÓŠ¹ŲČÜŅŗÖŠĪ¢Į£µÄĪļÖŹµÄĮæÅØ¶Č¹ŲĻµÕżČ·µÄŹĒ(Ė«Ń”)(””””)
A£®0.1 mol”¤L£1 CH3COONaČÜŅŗÓė0.1 mol”¤L£1 HClČÜŅŗµČĢå»ż»ģŗĻ£ŗc(Na£«)£½c(Cl£)£¾c(CH3COO£ )£¾c(OH£ )
B£®0.1 mol”¤L£1 NH4ClČÜŅŗÓė0.1 mol”¤L£1°±Ė®µČĢå»ż»ģŗĻ(pH£¾7)£ŗc(NH3”¤H2O)£¾c(NH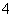 )£¾c(Cl£)£¾c(OH£ )
)£¾c(Cl£)£¾c(OH£ )
C£®0.1 mol”¤L£1 Na2CO3ČÜŅŗÓė0.1 mol”¤L£1 NaHCO3ČÜŅŗµČĢå»ż»ģŗĻ£ŗ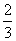 c(Na£«)£½c(CO
c(Na£«)£½c(CO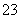 )£«c(HCO
)£«c(HCO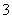 )£«c(H2CO3)
)£«c(H2CO3)
D£®0.1 mol”¤L£1 Na2C2O4ČÜŅŗÓė0.1 mol”¤L£1 HClČÜŅŗµČĢå»ż»ģŗĻ(H2C2O4ĪŖ¶žŌŖČõĖį): 2c(C2O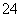 )£«c(HC2O
)£«c(HC2O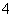 )£«c(OH£)£½c(Na£«)£«c(H£«)
)£«c(OH£)£½c(Na£«)£«c(H£«)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¢ŁČÜŅŗ”¢¢Ś½ŗĢ唢¢ŪŠü×ĒŅŗ”¢¢ÜČé×ĒŅŗŹĒĖÄÖÖ³£¼ūµÄ·ÖÉ¢Ļµ£¬¾²ÖĆ£¬·ÖÉ¢ÖŹŗĶ·ÖÉ¢¼ĮŗÜæģ·ÖĄė£¬ĘäÖŠ²śÉś³ĮµķµÄŹĒ__________£»·ÖÉ¢Ļµ·Ö²ćµÄŹĒ____________£»ÓĆŅ»ŹųĘ½ŠŠ¹āÕÕÉ䣬³öĻÖŅ»ĢõĆ÷ĮĮ”°¹āĀ·”±µÄŹĒ________£»ÄÜĶعż°ėĶøĤµÄŹĒ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠÓŠ¹ŲĪļÖŹ·ÖĄąŅ»¶ØÕżČ·µÄŹĒ(Ė«Ń”)(””””)
A£®Ēæµē½āÖŹ£ŗĀČ»ÆÄĘ”¢ĒāŃõ»Æ±µ”¢Ć÷·Æ
B£®Čõµē½āÖŹ£ŗ¼×Ėį”¢“æ¼ī”¢“×Ėįļ§
C£®·Ēµē½āÖŹ£ŗŅŗ°±”¢¶žŃõ»ÆĮņ”¢±½
D£®Ķ¬ĻµĪļ£ŗCH2O2”¢C2H4O2”¢C3H6O2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ»ÆŗĻĪļµÄĖ׳ĘÓė»ÆѧŹ½²»¶ŌÓ¦µÄŹĒ(””””)
A£®ĀĢ·Æ£FeSO4·7H2O
B£®Ć¢Ļõ£Na2SO4·10H2O
C£®Ć÷·Æ£ Al2(SO4)3·12H2O
D£®µØ·Æ£ CuSO4·5H2O
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¼×”¢ŅŅ”¢±ūČżÖÖĪļÖŹÖ®¼äÓŠČēĻĀ×Ŗ»Æ¹ŲĻµ£ŗ
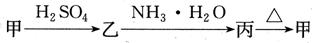
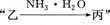 (1)Čō¼×ŗĶ±ū¶¼ŹĒ²»ČÜÓŚĖ®µÄ°×É«¹ĢĢåĪļÖŹ£¬
(1)Čō¼×ŗĶ±ū¶¼ŹĒ²»ČÜÓŚĖ®µÄ°×É«¹ĢĢåĪļÖŹ£¬ ¼ČÄÜČÜÓŚŃĪĖįÓÖÄÜČÜÓŚĒāŃõ»ÆÄĘČÜŅŗ”£Ōņ¼×ŹĒ £¬±ūŹĒ (Ģī»ÆѧŹ½)”£ Š“³ö
¼ČÄÜČÜÓŚŃĪĖįÓÖÄÜČÜÓŚĒāŃõ»ÆÄĘČÜŅŗ”£Ōņ¼×ŹĒ £¬±ūŹĒ (Ģī»ÆѧŹ½)”£ Š“³ö
×Ŗ»ÆµÄĄė×Ó·½³ĢŹ½£ŗ
(2)ČōŅŅČÜŅŗÖŠ¼ÓČėKSCNČÜŅŗ£¬ÓŠŗģÉ«³öĻÖ£¬Ōņ¼×ŹĒ £¬ ±ūŹĒ ”£(Ģī»ÆѧŹ½)”£Š“³ö”°¼×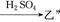 ×Ŗ»ÆµÄĄė×Ó·½³ĢŹ½£ŗ ”£
×Ŗ»ÆµÄĄė×Ó·½³ĢŹ½£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com