
ijͬѧ����ˮ�ʼ��վ����1000mL 1 mol��L��1NaOH��Һ�Ա�ʹ�á�
��1����ͬѧӦѡ��___________mL������ƿ��
��2���������������ͼ��ʾ������ͼ����Ӧ����ͼ�е� (��ѡ����ĸ)֮�䡣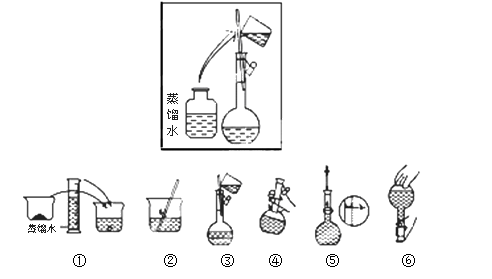
A������ۡ����� B������ڡ��� ���� C�������
��3����ͬѧӦ��������ƽ��ȡNaOH���� g��������Ϊ33.1 g���ձ�����������ƽ�ϳ�ȡ����NaOH����ʱ��������ͼ��ѡ������ȷ��ʾ����λ�õ�ѡ�� ����ѡ����ĸ����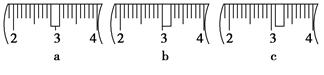
��4�����в�����������Һ��Ũ�ȴ�С�к�Ӱ�� (�ƫ����ƫС������Ӱ�족)��
�ٶ���ʱ�����Ӷ�����Ũ�Ȼ� ��
��ת����Һ�����У�����Һ�彦������Ũ�Ȼ� ��
������ƿδ���Ũ�Ȼ� ��
�ܶ���ҡ�Ⱥ�����Һ������ڿ̶��ߣ�Ũ�Ȼ� ��
��5��������Һ��ʵ�ʲ��������У�����Ҫ�죬�������� ����ʹ���Ƶ�NaOH��Һ��Ũ�ȱ�1 mol��L��1 �����С������
��1��1000��
��2��C��
��3��40.0�� c��
��4����ƫ�� ��ƫС ����Ӱ�� ����Ӱ��
��5��NaOH�׳��⡢�����տ����е�CO2�����ʣ����һ�㼴�ɣ��� С ��
���������������1������1000mL��Һֻ��ʹ��1000mL����ƿ����2��ͼ��Ϊ������ƿ�м�ˮ���������1��2����ʱ���ý�ͷ�ιܣ����Լ��ڢ�֮ǰ����3�����ݹ�ʽ��m=M��n n=C��V�ɵ�m=40.0g������ʱ����λ��Ϊ3.1��ѡC����4���ٶ���ʱ�����Ӷ�����ˮû�мӵ����ߣ�Ũ�Ȼ�ƫ��ת����Һ�����У�����Һ�彦������������ʧһ���֣�Ũ�Ȼ�ƫ�ͣ�������ƿδ������Ҫ��ˮ��û��Ӱ�죻�ܶ���ҡ�Ⱥ�����Һ������ڿ̶��ߣ��в�����Һ����ƿ����ƿ�ڣ�û��Ӱ�죬�����ټ�ˮ������Ũ�Ȼ��С����5��������Һ��ʵ�ʲ��������У�����Ҫ�죬�������ʱ�������Ƴ�������³���������Ũ�ȼ�С��
���㣺����һ�����ʵ���Ũ����Һ�����ơ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ��������90mL 2mol��L-̼������Һ��
��1�����Ƹ���Һʱ���������ʵ�����飬���������������õ�����_____����ѡ���
A. ������ƽ B. �ձ� C. ��ͷ�ι� D. 100ml����ƿ E. 90ml����ƿ F.������
��2��ʵ����������У�A.��ȡ̼���ƾ��壻B.��������ˮ���ձ�������ϴ��2��3�Σ�ÿ��ϴ��ҺҲת�Ƶ�����ƿ��C.���ձ��е���Һת�Ƶ�ѡ��������ƿ�У�D.��̼���ƾ��������ձ�����������ˮ�ܽ⣬���ò�����������ȣ�E.����õ�̼������Һװ���Լ�ƿ���ò����ñ�ǩ��F.������ƿ��ˮ���̶���1-2cm�����ý�ͷ�ιܵμ�ˮ��Һ����̶������У�G.����ƿ�����������µߵ�ҡ�ȡ�
�������������ȷ����˳���� B ����д��ĸ��
�ڱ�ʵ���ȡ��̼���ƾ���������� g
��������ʱ���ӿ̶��ߣ���������ҺŨ�� ���ƫ����ƫС������Ӱ�족��
���ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ����������� ����ѡ���
A. ��������Һ�壬ʹ��Һ����̶�������
B. С�ļ�������ƿ��Һ����������ʹ��Һ����̶�������
C. ��ȷ�������һ������Ũ����
D. ��������̼������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪���ᡢ��ˮ���ܶ�������ˮ���Ĺ�ϵ��ͼ��ʾ�����������백ˮ��һ�ݣ�����ݱ�����Ϣ���ش��������⣺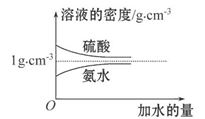
| | ���ʵ����ʵ��� Ũ��/mol��L-1 | ��Һ���ܶ� /g��cm-3 |
| ���� | c1 | ��1 |
| ��ˮ | c2 | ��2 |
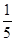 c2 mol��L-1�İ�ˮ��������ϣ�������Һ���ܶ�_____ ������ڡ�����С�ڡ����ڡ�����ͬ����2 g��cm-3��������Һ�����ʵ���Ũ��_____
c2 mol��L-1�İ�ˮ��������ϣ�������Һ���ܶ�_____ ������ڡ�����С�ڡ����ڡ�����ͬ����2 g��cm-3��������Һ�����ʵ���Ũ��_____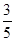 c2 mol��L-1�����Ϻ���Һ������仯���Բ��ƣ���
c2 mol��L-1�����Ϻ���Һ������仯���Բ��ƣ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣�
��1��������ͬ��O2��NH3��H2��Cl2���������У�����ͬ�¶Ⱥ���ͬѹǿ�����£���������� ��
��2�����������У����������ᷴӦ������������������Һ��Ӧ����_______��������ţ�
��NaAlO2 �� Ca(OH)2 ��Na2CO3 ��Al(OH)3
��3����ˮ�к��ж��ֳɷ֡�����ɫʯ����Һ������ˮ�У���Һ�Ժ�ɫ�����õijɷ���
����һ�������Һ��ɫ����ȥ�������õijɷ��� ��
��4����״���°�11.2L����ͨ��500ml0.8mol/LFeBr2��Һ�У�д����Ӧ��ȫ������ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���㣺
��1��0.3 mol NH3������������ԭ������_______NA��H2O������������ԭ������ȡ�
��2���ڱ�״����36 ��CO��CO2�Ļ����������Ϊ22.4L,��˻��������CO���ʵ���Ϊ ��CO2������Ϊ g ��
��3������״����224 L HCl��������635 mLˮ��(�ѣ�1 �磯cm3)������������ܶ�Ϊ1.18 �磯cm3�������������ʵ���Ũ��Ϊ ��
�����������С�����1λ��
��ijͬѧ����˲ⶨ����Ħ�������̽��ʵ�飬��������طֽ���O2��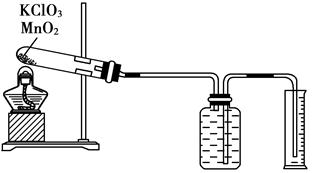
��1��ʵ�鲽�����£�
�����Ӻ�ʵ��װ�ã����װ�õ������ԡ�
�ڰ�����������ط�ĩ�������������̷�ĩ��Ͼ��ȣ����������Թ��У�ȷ�����Թܺ�ҩƷ��������Ϊ15.95 g��
�ۼ��ȣ���ʼ��Ӧ��ֱ���������������Ϊֹ��
�ܲ���������Ͳ��ˮ�����Ϊ285.0 mL�������T��,101kPa���������Ϊ360.0 mL��
��ȷ�����ԹܺͲ����������Ϊ15.55 g��
��2�������������ݣ���ʵ����T��,101kPa������Ħ�����Ϊ
�����������С�����1λ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͬ��ͬѹ�£���0.3molO2��0.2molO3�����ǵ�����֮��Ϊ ������������ԭ����֮��Ϊ �����ǵ����֮��Ϊ �����ǵ��ܶ�֮��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��1����Ũ��������ʵ���Ũ��Ϊ__________mol/L��
��2��ȡ����������ĸ�������Һʱ�������������в�����ȡ�� ���Ķ��ٶ��仯����__________��
A����Һ��H2SO4�����ʵ��� B����Һ��Ũ��
C����Һ��SO42������Ŀ D����Һ���ܶ�
��3��ijѧ����������Ũ���������ˮ����480 mL���ʵ���Ũ��Ϊ0.2 mol/Lϡ���ᡣ
�ٸ�ѧ����Ҫ��ȡ________mL����Ũ����������ơ�
������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�________________��
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳȷ��ȡ����Ũ���������������ر���ע��ʢ������ˮ��Լ30mL�����ձ��У��ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע��һ�����������ƿ��
D��������ƿ�ǽ����ߵ�ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�������
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1��2cm��
�������ƹ����У�����ʵ�����ʹ�����Ƶ�ϡ��������ʵ���Ũ��ƫ�ߵ���_________
A������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ��
B��ϡ���õ��ձ��Ͳ�����δϴ��
C��ϴ��������ƿδ�����������������Һ
D����Һע������ƿǰû�лָ������¾ͽ��ж���
E������ʱ���ӹ۲찼Һ��
F����ˮ�����̶��ߺ��ý�ͷ�ι����������Һ��
���ֽ�100mL��������300mL 0.4mol/LCuSO4��Һ��ϣ�����仯���Բ��ƣ�������Һ��SO42�������ʵ���Ũ����_________mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������գ�
��1����SO2��SO3�з��Ӹ�����Ϊ1��1 ʱ��ԭ������֮��Ϊ ������֮��Ϊ ��
��2���кͺ�0.2 mol HCl��ϡ���ᣬ��NaOH������Ϊ g��
��3������m gij���壬����˫ԭ�ӷ��ӹ��ɣ�����Ħ������ΪM g��mol-1����
�ٸ���������ʵ���Ϊ mol��
�ڸ������ڱ�״���µ����Ϊ L��
�۸���������ˮ���γ�V L��Һ�������Ƿ�Ӧ��������Һ�����ʵ���Ũ��Ϊ mol/L��
��4����5mol/L��Mg(NO3)2��Һa mLϡ����b mL��ϡ�ͺ���Һ��NO3-�����ʵ���Ũ���� mol/L��
��5���õ������0.1mol/L��BaCl2��Һ����ʹ��ͬ�����Fe2(SO4)3��Na2SO4��KAl(SO4)2������Һ�е�SO42-ǡ����ȫ�����������������ε����ʵ���Ũ��֮��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��0.5 mol H2O������Ϊ_______________�����к���_______________��ˮ���ӣ�����_______________��ԭ�ӡ�
��2����������50g��HCl��NH3��CO2��O2���������У����з�����Ŀ���ٵ���_______________������ͬ�¶Ⱥ���ͬѹǿ�����£����������_______________�������С����_______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com