
��.������ʳ����ȷ��ҩ�������彡������Ҫ��֤��
����������������Aʳ�Σ�BС�մ�Cƻ��֭��D�����ǣ�E��ù�أ��밴����Ҫ�����(�����)��
����ά����C���� ����ֱ�ӽ���ѪҺ�������������� ��Ӧ����㷺�Ŀ�����֮һ���� ���ȿ���Ϊ���ɼ����ֿ�����θ�������� ��ʳ�ù��������Ѫѹ���ߡ���������� ��
��.������������ʹ�����������������������һ����Ҫ��־��
��1��д����ҵ���ó�������������Ҫ��ѧ��Ӧ����ʽ�� ��
��2�������˵�����δ��ʱϴ������Һ�к�NaCl��,�ڶ�������ʴ���ֺ��ɫ��ߡ��Իش�
�������ĸ�ʴ��Ҫ���� ���ѧ��绯ѧ����ʴ��ɵġ��γɵ��������Ҫ�ɷ��� ��
��Ϊ��ֹ�ִ��Ĵ����ں�ˮ�и�ʴ��һ���ڴ������� ���п�顱��ͭ�顱����
��.������������������ͷ�չ����Ҫ���ʻ���������ʹ�ò��Ͽ��Ը������ǵ����
��1���������ݽ���������������ϡ����в��ϲ����ڹ����β��ϵ��� ������ĸ����
A��ʯ��ʯ B��ˮ�� C������
��2�������в����У��������ǽ������ϵ��� (����ĸ)������������Ʒ���� ��
A�����ڡ������� B��������ϩ���ϡ���C���������մ� D��������
��3�������йغϽ����ʵ�˵����ȷ���� ������ĸ����
A���Ͻ���۵�һ������ijɷֽ�����
B���Ͻ��Ӳ��һ������ijɷֽ�����
C����ɺϽ��Ԫ��������ͬ���Ͻ�����ܾ�һ����ͬ
D���Ͻ�����ɷֽ�����ȣ�����������������������ѧ���е����
��4���ϳ����ϡ��ϳ��� �dz�˵������ϳɲ��ϡ�
��.C D E B A
��.��1��Fe2O3+3CO 2Fe+3CO2����Ӧ�������ԣ�����ƽ��1�֣�
2Fe+3CO2����Ӧ�������ԣ�����ƽ��1�֣�
��ֻҪд������Ӧ���֣����д̿��������������̼��̿�ķ�Ӧ���۷֣�
��2�� �ٵ绯ѧ Fe2O3�� Fe2O3?nH2O ��п��
��. ��1��A ��2��C B ��3��D ��4���ϳ���ά
���������������. ����ά����C����ƻ��֭����ֱ�ӽ���ѪҺ�������������������ǣ�Ӧ����㷺�Ŀ�����֮һ������ù�أ��ȿ���Ϊ���ɼ����ֿ�����θ��������С�մ�ʳ�ù��������Ѫѹ���ߡ����������ʳ�Ρ���. ��1����ҵ���ó�������Ҫ�ɷ�����������������������Ҫ��ѧ��Ӧ����ʽ��Fe2O3+3CO 2Fe+3CO2����2���������ĸ�ʴ��Ҫ���� �ٵ绯ѧ��ʴ��ɵġ��γɵ��������Ҫ�ɷ���Fe2O3�� Fe2O3?nH2O����ͨ������ԭ���װ�ÿ��Ա����ִ��Ĵ����ں�ˮ�и�ʴ��һ���ڴ�������п�飬����п�������ã��γ�ԭ�����п����������. ��1�������ڹ����β��ϵ���A��ʯ��ʯ����2�������в����У��������ǽ������ϵ���C���������մɡ�����������Ʒ����B��������ϩ���ϡ���3���Ͻ������У��Ͻ���۵�һ������ijɷֽ����ͣ��Ͻ��Ӳ��һ������ijɷֽ����ߣ���ɺϽ��Ԫ��������ͬ�������ڲ��ṹ��ͬ�Ͻ������Ҳ���ܲ���ͬ���Ͻ�����ɷֽ�����ȣ�����������������������ѧ���е���ܡ���4���ϳ����ϡ��ϳ��ͺϳ���ά�dz�˵������ϳɲ��ϡ�
2Fe+3CO2����2���������ĸ�ʴ��Ҫ���� �ٵ绯ѧ��ʴ��ɵġ��γɵ��������Ҫ�ɷ���Fe2O3�� Fe2O3?nH2O����ͨ������ԭ���װ�ÿ��Ա����ִ��Ĵ����ں�ˮ�и�ʴ��һ���ڴ�������п�飬����п�������ã��γ�ԭ�����п����������. ��1�������ڹ����β��ϵ���A��ʯ��ʯ����2�������в����У��������ǽ������ϵ���C���������մɡ�����������Ʒ����B��������ϩ���ϡ���3���Ͻ������У��Ͻ���۵�һ������ijɷֽ����ͣ��Ͻ��Ӳ��һ������ijɷֽ����ߣ���ɺϽ��Ԫ��������ͬ�������ڲ��ṹ��ͬ�Ͻ������Ҳ���ܲ���ͬ���Ͻ�����ɷֽ�����ȣ�����������������������ѧ���е���ܡ���4���ϳ����ϡ��ϳ��ͺϳ���ά�dz�˵������ϳɲ��ϡ�
���㣺���⿼�黯ѧ�������������ѧ������֪ʶ��


 �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ѧ����ѡ��2��ѧ�뼼������15�֣�
��Ca3(PO4)2��SiO2����̿��Ϊԭ�������轺��SiO2��nH2O�����ס����ἰCH3OH�����й��չ���ԭ���ۺ������ʸߣ��������١�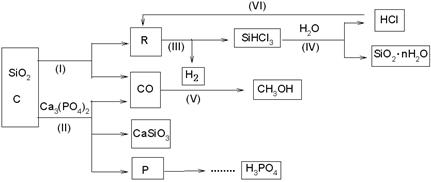
��1��������Ӧ�У������û���Ӧ���� [ѡ���������������������������]
��2�������½��еķ�Ӧ��Ļ�ѧ����ʽΪ�� ������
���������CaSiO3������ ���� ������
��3����Ӧ�����ڸ�����������ˮ�����½��У���ԭ���� ��
��4��CH3OH������ȼ�ϵ�ص�ȼ�ϣ���ǿ���Խ����У������ĵ缫��ӦʽΪ ��
��5��ָ����VI����һ�����ڹ�ҵ�����ϵ����� ���� ����
��6��д����P��H3PO4�йط�Ӧʽ
�� ���� ����
�� ���� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ⷨ�ڽ�������������������������ˮ������ʮ����Ҫ�����á�
��1����ͼΪ��⾫������ʾ��ͼ��________����a��b����Ϊ�������ʵĴ�������b������������ɫ�������ɣ������ɸ�����ĵ缫��ӦʽΪ________________________��
AgNO3��HNO3��Һ
��2����ⷨ�������Ժ�����ˮ����Ҫ����Cr2O72-��ʱ�����������������������������д��ڷ�ӦCr2O72-��6Fe2����14H��=2Cr3����6Fe3����7H2O�����Cr3����Cr��OH��3��ʽ��ȥ���ش��������⣺
��д���缫��Ӧʽ������________________������________________��
�ڵ�����1 mol Cr��OH��3ʱ����·��ת�Ƶ��ӵ����ʵ�������Ϊ________mol��
�۵���������Fe��OH��3�������ɣ�ԭ����_________________________________________________________________
________________________________________________________________��
��3����⽵�ⷨ����������ˮ�������ε���Ⱦ����⽵��NO3-��ԭ����ͼ��ʾ��
�ٵ�Դ����Ϊ________����A��B����������ӦʽΪ________________________________________________________________��
������������ת����2 mol���ӣ���Ĥ������Һ�������仯���m������m����Ϊ________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ȤС���ͬѧ����ͼ��ʾװ���о��йص绯ѧ������(�ס��ҡ�����������������)�����պϸ�װ�õĿ���Kʱ���۲쵽�����Ƶ�ָ�뷢����ƫת��
�������� ���׳ء������������ҳء���������������
��ش��������⣺
(1)�ס��ҡ���������Ϊԭ��ص���________(��׳ء����ҳء����ء�)��
(2)������F�缫Ϊ________(�������������������������������)���óص��ܷ�ӦʽΪ__________________________________________________��
(3)���ҳ���C�缫��������10.8 gʱ���׳���B�缫����������O2�����Ϊ________mL(��״��)��
(4)һ��ʱ��Ͽ�����K������������ʹ���ػָ�����ӦǰŨ�ȵ���________(��ѡ����ĸ)��
| A��Cu������������ | B��CuO | C��CuCO3 | D��Cu2(OH)2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ס��ҵ�ʵ��װ����ͼ��ʾ���������ֱ����ȼҵ����ʾ��ͼ���Ʊ������ѵ�ʾ��ͼ��
��ش��������⣺
(1)д����װ����̼������ĵ缫��Ӧʽ��_______________________________��
(2)��֪��5Cl2��I2��6H2O=10HCl��2HIO3������ʪ��ĵ���KI��ֽ������װ���е�̼������������Ϊ________________________________������װ����ת��0.02 mol���Ӻ�ֹͣʵ�飬�ձ�����Һ�����Ϊ200 mL�����ʱ��Һ��pH��________��(���������£��Ҳ����ǵ���������Ӧ)
(3)��ҵ�Ͼ����õ����ӽ���Ĥ�����ӽ���Ĥ�������ӽ���Ĥ�������ӽ���Ĥ���֣������ӽ���Ĥֻ����������ͨ���������ӽ���Ĥֻ����������ͨ��������װ���еķ�Ӧ���ڹ�ҵ����ʱ��Ϊ����ֹ��������֮��ķ�Ӧ��ͨ�������ͼ��ʾ��װ�ã�Na�����ƶ�������ͼ�б�ע����H2�ij�����________(�C������D������E����F��)��________(��ܡ����ܡ�)�������ӽ���Ĥ���������ӽ���Ĥ��
(4)�о����֣�������ʯī������������������������CaO������ʣ����ö�װ�û�ý����ƣ����Ը�Ϊ��ԭ������ԭ���������Ʊ������ѡ�
��д�������ĵ缫��Ӧʽ��___________________________________________��
�����Ʊ�������ǰ��CaO���������䣬��ԭ����(���ϻ�ѧ�������)__________________________________________________________��
�۵��������趨�ڸ����������ϵ�ԭ����____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij����С��ֱ�����ͼ��ʾװ�ö�ԭ��غ͵���ԭ������ʵ��̽����

ͼ1 ͼ2
��ش�:
��.��ͼ1��ʾװ�ý��е�һ��ʵ�顣
(1)�ڱ�֤�缫��Ӧ����������,�������Cu���缫����������(����ĸ���)��
| A���� | B��ʯī | C���� | D���� |
 ������������(��������ҡ�������������)�ƶ�;��ֽ���ܹ۲쵽����������������������������������������������
������������(��������ҡ�������������)�ƶ�;��ֽ���ܹ۲쵽����������������������������������������������  )����Һ�г��Ϻ�ɫ��
)����Һ�г��Ϻ�ɫ�� Fe
Fe +4H2O����������������
+4H2O����������������  Fe2O3+ZnO+2K2ZnO2
Fe2O3+ZnO+2K2ZnO2�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͼװ����ʾ��C��D��E��F���Ƕ��Ե缫���ס�������Һ�������Ũ�ȶ���ͬ(����ͨ��ǰ����Һ�������)��A��BΪ���ֱ����Դ����������ֱ����Դ��ͨ��F�������ʺ�ɫ����ش�
��1�� һ��ʱ�������Һ��ɫ ��
д������C�ĵ缫��Ӧʽ ��
��2�� ���ס���װ���е�C��D��E��F�缫��ֻ��һ�ֵ�������ʱ����Ӧ���ʵ����ʵ���֮��Ϊ ��
��3�� ���ñ�װ�ø�ͭ�����������Һ�� ��Һ����������Һ��pH��13ʱ(��ʱ����Һ���Ϊ500 mL)�����жƼ���������������Ϊ ��
��4�� ����F�缫����Ϊ��������װ�ö����䣬�����з����ܷ�Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧ��ȤС��Ϊ��̽�����缫��ԭ����е�����,��Ʋ�����������һϵ��ʵ��,ʵ������¼����:
| ��� | �缫���� | �������Һ | ������ָ�� ƫת���� |
| 1 | Mg��Al | ϡ���� | ƫ��Al |
| 2 | Al��Cu | ϡ���� | ƫ��Cu |
| 3 | Al��ʯī | ϡ���� | ƫ��ʯī |
| 4 | Mg��Al | NaOH��Һ | ƫ��Mg |
| 5 | Al��Zn | Ũ���� | ƫ��Al |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��109g5.51����NaOH��Һ��������CuSO4��Һ��200g10.00����K2SO4��Һ���缫��Ϊʯī�缫��
��ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47��������c�缫�������ӡ��ݴ˻ش����⣺
��1���缫b�Ϸ����ĵ缫��ӦΪ___________________________________��
��2���缫b�����ɵ������ڱ�״���µ����Ϊ__________________����ʱ���ձ���NaOH��Һ�����ʵ���Ũ��Ϊ������Һ���ܶ�Ϊ1g/cm3��_______________��
��3���缫c�������仯��___________g����ʹ�������еĵ��Һ�ָ�����ʼ״̬��Ӧ������Һ�м���������___________������ĸ��ţ���
| A��Cu(OH)2 | B��Cu2O | C��CuCO3 | D��Cu2(OH)2CO3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com