
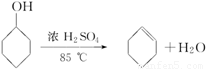
��ѧС���������������������װ��(����ͼ)���û������Ʊ�����ϩ��
��֪��
|
|
�ܶ�(g/cm3) |
�۵�(��) |
�е�(��) |
�ܽ��� |
|
������ |
0.96 |
25 |
161 |
������ˮ |
|
����ϩ |
0.81 |
��103 |
83 |
������ˮ |
(1)�Ʊ���Ʒ
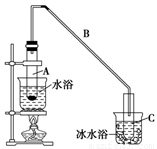
��12.5 mL�����������Թ�A�У��ټ���1 mLŨ���ᣬҡ�Ⱥ�������Ƭ����ֹ���У���������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
�ٵ���B���˵�������е�������________��
���Թ�C���ڱ�ˮԡ�е�Ŀ����______________________________��
(2)�Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��________��(��ϡ����¡�)����Һ����________(������)ϴ�ӡ�
A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ
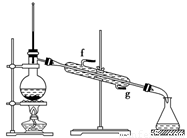
���ٽ�����ϩ����ͼװ��������ȴˮ��________�ڽ���(�g����f��)������ʱҪ������ʯ�ң���Ŀ����_____________________________________��
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��________���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����________��
A������ʱ��70 �濪ʼ�ռ���Ʒ
B��������ʵ����������
C���Ʊ���Ʒʱ���������Ʒһ������
(3)�������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������________��
A���ֱ�������Ը��������Һ
B���ֱ�����ý�����
C���ֱ�ⶨ�е�
��9�֣���1�����������ڷ�ֹ����ϩ�Ļӷ�
��2�����ϡ�C����g����ȥˮ�֡���83 �桡C ��3��BC
��������
�����������1���������ɵĻ���ϩ�ķе�Ϊ83�棬Ҫ�õ�Һ̬����ϩ������B���˵���������������ã����ڻ���ϩ���������ڻ���ϩ�е�ͣ��ӷ������Ա�ˮԡ��Ŀ�Ľ����¶ȣ���ֹ����ϩ�Ļӷ���
��2���ٻ���ϩ���ܶ�С��ˮ�ģ�������ˮ���������ϲ㣻���ڷ�Һ��ϩ��Ʒ�л�������������ͻ������������Ʊ����������ᴿ����ʱ�ñ���Na2CO3��Һϴ�ӿɳ�ȥ��ʹ�������ѡ���ˮ̼������Һ�����������Ը�����أ��������������ϩ����ѡC����ʯ�Ҿ�����ˮ�ԣ���������ʱҪ������ʯ�ҵ�Ŀ���dz�ȥˮ�֡�
����ȴˮ�������������������෴�ģ������ȴˮ��g�ڽ��룬f�ڳ�����
���ռ�ʱ��ֻҪҺ���¶ȴﵽ����ϩ�ķе㣬�ܰ�����������Ϳ����ˣ��¶�̫���������¶�̫�ߣ����·�Ӧ�¶ȹ��߶�̼������˸��ݻ���ϩ�ķе��֪��һ�������83�����ң���ʵ���ƵõĻ���ϩ��Ʒ�����������۲�������˵����Ӧ���ת���ʵͣ���˿��ܵ�ԭ�����Ʊ���Ʒʱ���������Ʒһ����������ѡC��
��3�������Ʒ�뾫Ʒ�ɼ�������ƣ��۲��Ƿ�������������������壬���Ǿ�Ʒ�������û�й̶��ķе㣬���������й̶��ķе㣬ͨ���ⶨ����ϩ��Ʒ�ͻ���ϩ��Ʒ�ķе㣬���жϲ�Ʒ�Ĵ��ȣ���˴�ѡBC��
���㣺�����л����Ʊ����й�ʵ��̽��
�������������л��ϳ�Ϊ�����ۺϿ�����ʵ�����ƻ���ϩ��֪ʶ�ʹ����������ʣ�������ѧ���ۺ�����ʵ��������������е��Ѷȵ����⡣����ʱע�����ʵ��ԭ���ͷ������ر���ʵ��Ļ���������ѧϰ��ע����ۡ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| �ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� | ������ | 0.96 | 25 | 161 | ������ˮ | ����ϩ | 0.81 | -103 | 83 | ������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


| �ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� | ������ | 0.96 | 25 | 161 | ������ˮ | ����ϩ | 0.81 | -103 | 83 | ������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
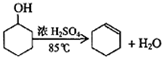

| �ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� | |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ÷������������ѧ�߶�5���¿���ѧ�Ծ����������� ���ͣ�ʵ����
��20�֣�ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ
��1���Ʊ���Ʒ
��12.5mL�����������Թ�A�У��ټ���lmLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ��������____________������B���˵�������е�������____ ��
���Թ�C���ڱ�ˮԡ�е�Ŀ����_____________________________��
��2)�Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��______��(���ϻ���)����Һ����_________ (������)ϴ�ӡ�
A��KMnO4��Һ B��ϡH2SO4 C��Na2CO3��Һ
���ٽ�����ϩ����ͼװ��������ȴˮ��_________�ڽ��롣����ʱҪ������ʯ�ң�Ŀ����______________ ____��
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��_________���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ����____________________
A������ʱ��70�濪ʼ�ռ���Ʒ
B��������ʵ����������
C���Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������_________��
A�������Ը��������Һ B���ý����� C���ⶨ�е�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����������ѧ�߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ��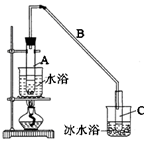
��֪��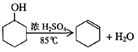
| | �ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� |
| ���Ѵ� | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | ��103 | 83 | ������ˮ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com