
| A�� | ����NH4+��Cl-��H+��OH-����Һ�У�����Ũ��һ����c��Cl-����c��NH4+����c��OH-����c��H+�� | |
| B�� | �����£�pH=6�Ĵ���������ƵĻ����Һ�У�c��Na+����c��CH3COO-�� | |
| C�� | ��0.2 mol•L-1 CH3COOH��Һ��0.1 mol•L-1 NaOH��Һ�������ϣ���Ӧ��Ļ����Һ�У�2c��OH-��+c��CH3COO-���T2c��H+��+c��CH3COOH�� | |
| D�� | ������pH=6��NH4Cl��Һ�У�c��Cl-����c��NH4+����c��NH3•H2O����c��H+����c��OH-�� |
���� A������NH4+��Cl-��H+��OH-����Һ�����ʿ���ΪNH4Cl��
B�������£�pH=6�Ĵ���������ƵĻ����Һ�����ԣ����������ڴ��������ˮ�⣻
C����0.2 mol•L-1 CH3COOH��Һ��0.1 mol•L-1 NaOH��Һ�������ϵõ���Ũ�ȵĴ���ʹ����ƣ���Һ�д��ڵ���غ�������غ���������
D��������pH=6��NH4Cl��Һ��笠�����ˮ�������ԣ�
��� �⣺A������NH4+��Cl-��H+��OH-����Һ�У�������ΪNH4Cl����Һ������Ũ�ȴ�СΪ��c��Cl-����c��NH4+����c��H+����c��OH-��������Ũ�Ȳ�һ����c��Cl-����c��NH4+����c��OH-����c��H+������A����
B�������£�pH=6�Ĵ���������ƵĻ����Һ�����ԣ����������ڴ��������ˮ�⣬c��Na+����c��CH3COO-������B����
C����0.2 mol•L-1 CH3COOH��Һ��0.1 mol•L-1 NaOH��Һ�������ϵõ���Ũ�ȵĴ���ʹ����ƣ���Һ�е���غ�c��OH-��+c��CH3COO-���Tc��H+��+c��Na+���������غ�c��CH3COO-��+c��CH3COOH��=2c��Na+����2c��OH-��+c��CH3COO-���T2c��H+��+c��CH3COOH������C��ȷ��
D��������pH=6��NH4Cl��Һ��笠�����ˮ�������ԣ�����ˮ�ĵ���ƽ�⣬c��Cl-����c��NH4+����c��H+����c��NH3•H2O����c��OH-������D����
��ѡC��
���� ���⿼��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ�ε�ˮ��ԭ������Ӱ��Ϊ���ؼ���ע�����յ���غ㡢�����غ㡢�����غ�ĺ��弰���ж�����Ũ�ȴ�С�е�Ӧ�÷���������������ѧ�������Ӧ�û���֪ʶ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
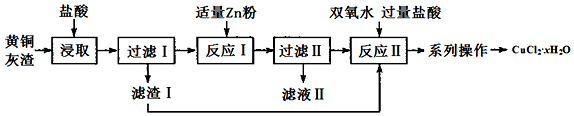
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ͬ���ʵ�����ʯī����ʯ��ȣ����ʯ������������ | |
| B�� | ���ʯת��Ϊʯī�Ƿ��ȷ�Ӧ�����ʯ��ʯī�ȶ� | |
| C�� | ���ʯת��Ϊʯī�����ȷ�Ӧ��ʯī�Ƚ��ʯ�ȶ� | |
| D�� | ����Ϊͬλ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C3H6�� C5H10 | B�� | C3H4�� C4H6 | C�� | C2H6�� C8H18 | D�� | C2H6�� C3H6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NO2��NO | B�� | NO2��CO2 | C�� | N2��O2 | D�� | O2��CO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ͨ��H2S��Һ�У��г������� | |
| B�� | �Ա������������ˮ�е��ܽ��� | |
| C�� | һ�����������ֱ������ȷ�Ӧ���Ƚ�������������̬ | |
| D�� | �Ƚ�������Ԫ�ص�ԭ�ӽṹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʽΪC5H11Cl���л�������к�2������ͬ���칹����4�� | |
| B�� | �ױ��������ڹ����·�Ӧ��Ҫ����2��4--���ȼױ� | |
| C�� | ���ӡ���ȩͨ���Ӿ۷�Ӧ���Ƶ÷�ȩ��֬ | |
| D�� | �ϳ�˳���� 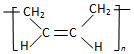 ���ĵ�����CH3-CH=CH-CH3 ���ĵ�����CH3-CH=CH-CH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ˮ�����ӻ����� | |
| B�� | pH=7����Һһ�������� | |
| C�� | NaHSO4 ��Һһ�������� | |
| D�� | ������������Һ�ζ�������Һ���ζ��յ�ʱ�������Һ��һ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A������γ� | B����θ����� | C�п���ƻ����ù | D���ű��� |
 |  |  |  |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com