
���� ��1����NH4Cl��ʾ���ԣ�
��CH3COONH4��ʾ���ԣ�
��NH4HSO4��ʾ���ԣ�
��Ũ�Ⱦ�Ϊ0.1mol/L��NH3•H2O��NH4Cl���Һ��һˮ�ϰ��������笠����ӵ�ˮ�⣬��Һ�Լ��ԣ�
�ݰ�ˮ��NH3?H2O����ʾ���ԣ���������������笠�����Ũ����С��
��Һ��笠�����ˮ��̶Ⱥ�������ʵ���̶ȷ����Ƚ���ҺPH��
��2������������֪笠�����Ũ�ȴ�С��
��3��������淋�������笠����ӡ������Ӻ���������ӣ�
��4��Ũ�Ⱦ�Ϊ0.1mol/L��NH3•H2O��NH4Cl���Һ�У�һˮ�ϰ��������笠����ӵ�ˮ�⣬��Һ�Լ��ԣ��ݴ˱Ƚ�����Ũ�ȴ�С��
��� �⣺��1����NH4Cl��ʾ���ԣ�
��CH3COONH4��ʾ���ԣ�
��NH4HSO4��ʾ���ԣ�
��Ũ�Ⱦ�Ϊ0.1mol/L��NH3•H2O��NH4Cl���Һ��һˮ�ϰ��������笠����ӵ�ˮ�⣬��Һ�Լ��ԣ�
�ݰ�ˮ��NH3?H2O����ʾ���ԣ���������������笠�����Ũ����С��������淋�����������ӻ�����笠����ӵ�ˮ�⣬��������ӻ�ٽ�笠����ӵ�ˮ�⣬����������Һ��c��NH4+���ɴ�С��˳���Ǣܢۢ٢ڢݣ���ҺPH��СΪ�ݢܢڢ٢ۣ�pH��С���Ǣۣ�
�ʴ�Ϊ���ۣ��ݣ�
��2�����ݣ�1���ķ�����֪笠�����Ũ�ȴ�СΪ���ܢۢ٢ڢݣ���Һ�١��ڡ�����c��NH4+���Ĵ�С˳���Ǣۢ٢ڣ�
�ʴ�Ϊ���ۢ٢ڣ�
��3��������淋�������笠����ӡ������Ӻ���������ӣ����뷽��ʽΪ��NH4HSO4�TNH4++H++SO42-��
�ʴ�Ϊ��NH4HSO4�TNH4++H++SO42-��
��4��Ũ�Ⱦ�Ϊ0.1mol/L��NH3•H2O��NH4Cl���Һ�У�һˮ�ϰ��������笠����ӵ�ˮ�⣬��Һ�Լ��ԣ���Һ������Ũ�ȴ�СΪ��c��NH4+����C ��Cl-����C��OH-����C��H+����
�ʴ�Ϊ��c��NH4+����C ��Cl-����C��OH-����C��H+����
���� ���⿼��ѧ���ε�ˮ��ԭ����Ӧ���Լ�����Ũ�ȴ�С�Ƚϵȷ����֪ʶ�������ۺ�֪ʶ�Ŀ��飬�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��þ3%-5%��þ���Ͻ����ѳ�Ϊ�ִ����졢������������е�������ҵ����Ҫԭ���ϣ�����һ����֪����Ϊm1g��þ���Ͻ����ⶨ����þ��������������λͬѧ������������ֲ�ͬ��ʵ�鷽����
��þ3%-5%��þ���Ͻ����ѳ�Ϊ�ִ����졢������������е�������ҵ����Ҫԭ���ϣ�����һ����֪����Ϊm1g��þ���Ͻ����ⶨ����þ��������������λͬѧ������������ֲ�ͬ��ʵ�鷽�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������Ȼ�����Һ��ϣ�Fe+Fe3+=2Fe2+ | |
| B�� | �Ȼ�����Һ�백ˮ��Ӧ��Al3++3OH-=Al��OH��3�� | |
| C�� | ̼�����ϡ���ᷴӦ��CO32-+2H+=H2O+CO2�� | |
| D�� | ����������������Һ��Ӧ��CH3COOH+OH-=CH3COO-+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ˮ���ȣ�ƽ���������ƶ���Kw���� | |
| B�� | ��ˮ�м�������NaOH���壬ˮ�����c��OH-������ | |
| C�� | ��ˮ�м�������CH3COONa���壬ƽ���������ƶ���Kw���� | |
| D�� | ��ˮ�м�������NaHSO4���壬ˮ�ĵ���ƽ���������ƶ���c��H+������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
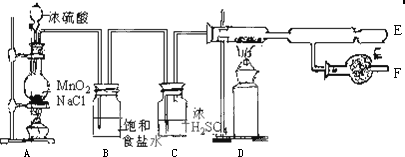
 ��CH4��Ƴ�ȼ�ϵ�أ��������ʸ��ߣ�װ����ͼ��ʾ��A��BΪ���̼����������ͨ����飬�ڱ�״���£����ļ�������ΪV L��
��CH4��Ƴ�ȼ�ϵ�أ��������ʸ��ߣ�װ����ͼ��ʾ��A��BΪ���̼����������ͨ����飬�ڱ�״���£����ļ�������ΪV L���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ������ɴ��ķ���ʽΪC10H17O | |
| B�� | ������ɴ���NaOH����Һ�пɷ�����ȥ��Ӧ | |
| C�� | �����ط����к���7������̼ԭ�� | |
| D�� | ���������ȵ��ᡢ����Һ�о����ȶ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Ļ�ѧʽ��HClO2 | B�� | �����ӵĽṹʾ��ͼ�� | ||
| C�� | ������Ϊ127�ı�ԭ�ӣ�127Ba | D�� | ������NH3���е�Ԫ�صĻ��ϼۣ�+3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ʯұ���� | B�� | ��ʳ�����ůˮƿ�е�ˮ�� | ||
| C�� | ��ú����ˮú�� | D�� | �ɱ�����ͨ�紦��Ȼ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com