
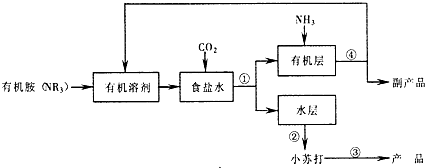
| ||
| ||
| ||
| ||



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ¼×”¢ŅŅ”¢±ū”¢¶””¢ĪģĪåÖÖĒ°ĖÄÖÜĘŚŌŖĖŲ£¬ĘäÖŠ¼×ŅŅ±ū¶¼ĪŖ·Ē½šŹōŌŖĖŲ£¬ŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆ¹ŲĻµČēĶ¼1ĖłŹ¾£®¶””¢ĪģĮ½ÖÖŌŖĖŲŌņĪ»ÓŚĶ¬Ņ»ÖÜĘŚĶ¬Ņ»×壬¶ųĒŅĪģµÄŌ×ÓŠņŹż±Č¶”“ó2£®
¼×”¢ŅŅ”¢±ū”¢¶””¢ĪģĪåÖÖĒ°ĖÄÖÜĘŚŌŖĖŲ£¬ĘäÖŠ¼×ŅŅ±ū¶¼ĪŖ·Ē½šŹōŌŖĖŲ£¬ŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆ¹ŲĻµČēĶ¼1ĖłŹ¾£®¶””¢ĪģĮ½ÖÖŌŖĖŲŌņĪ»ÓŚĶ¬Ņ»ÖÜĘŚĶ¬Ņ»×壬¶ųĒŅĪģµÄŌ×ÓŠņŹż±Č¶”“ó2£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
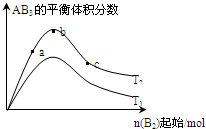
| A”¢·“Ó¦ĖŁĀŹa£¾b£¾c |
| B”¢ČōT2£¾T1£¬ŌņÕż·“Ó¦Ņ»¶ØŹĒĪüČČ·“Ó¦ |
| C”¢“ļµ½Ę½ŗāŹ±£¬AB3µÄĪļÖŹµÄĮæ“óŠ”ĪŖ£ŗb£¾c£¾a |
| D”¢“ļµ½Ę½ŗāŹ±A2µÄ×Ŗ»ÆĀŹ“óŠ”ĪŖ£ŗb£¾a£¾c |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
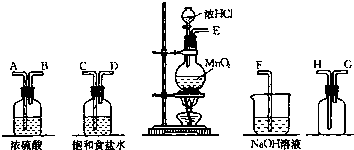
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
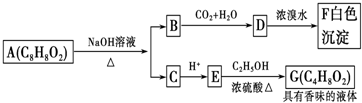
| A”¢øł¾ŻĶ¼Ź¾æÉĶĘÖŖDĪŖ±½·Ó |
| B”¢GµÄĶ¬·ÖŅģ¹¹ĢåÖŠŹōÓŚõ„ĒŅÄÜ·¢ÉśŅų¾µ·“Ó¦µÄÖ»ÓŠŅ»ÖÖ |
| C”¢ÉĻŹöø÷ĪļÖŹÖŠÄÜ·¢ÉśĖ®½ā·“Ó¦µÄÓŠA”¢B”¢D”¢G |
| D”¢AµÄ½į¹¹ÖŠŗ¬ÓŠĢ¼Ģ¼Ė«¼ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
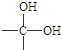 ”ś
”ś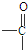 +H2O
+H2O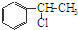 Ӣ
”¢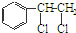 µČ¶¼ŹĒAŗĶCl2·¢Éś·“Ӧɜ³ÉµÄ²śĪļ£¬EŹĒŅ»ÖÖøß·Ö×Ó»ÆŗĻĪļ£¬Ķø¹āŠŌÄÜŗĆ£¬³£ÓĆ×÷Ņ»Š©µĘŹĪĶāæĒ£®¹ż³ĢÖŠŅ»Š©Š”·Ö×Ó¶¼ŅŃ¾ĀŌČ„£®
µČ¶¼ŹĒAŗĶCl2·¢Éś·“Ӧɜ³ÉµÄ²śĪļ£¬EŹĒŅ»ÖÖøß·Ö×Ó»ÆŗĻĪļ£¬Ķø¹āŠŌÄÜŗĆ£¬³£ÓĆ×÷Ņ»Š©µĘŹĪĶāæĒ£®¹ż³ĢÖŠŅ»Š©Š”·Ö×Ó¶¼ŅŃ¾ĀŌČ„£®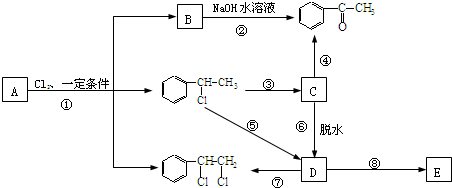
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com