
| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN |
| pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 |
| ���� | Fe 2+ | Cu2+ | Mg2+ |
| pH | 7.6 | 5.2 | 10.4 |
���� ��1��Խ����ˮ������е������ӣ�Խ����������ӣ����ε�ˮ����ɣ�Խ��Խˮ�����ش���ĵ�����ɣ�����Խǿ��ϡ�ͺ�pH�仯Խ��
��2����̼������ӵ�ˮ��̶ȴ���̼��������ӣ�����ˮ��ԭ���������Ӷ�ˮ�ĵ���ƽ��Ĵٽ����жϣ�
�ڸ��ݵ���غ㣺��Һ�������ӵ����ʵ���Ũ��֮�͵��������Ӻ�������Ũ��֮�ͣ��ݴ˻ش�
��3��̼���������ˮ������̼����Ӻ����������ӣ�0.1mol•L-1NaHCO3��Һ��pH����8��˵��̼��������ӵ�ˮ��̶ȴ��ڵ���̶ȣ�
��4���ٸ��������ˮ��͵���غ���������ע�������ˮ�������ģ�
�ڿ�ʼ����ʱpHС�������ȳ������ܶȻ�����ԽС������Խ�ȳ�����0.2mol/L��CuSO4��Һ��Cu2+������Ϊ��ȫ��ʹCu2+Ũ�Ƚ���ԭ����ǧ��֮һ����Ӧ����Һ���NaOH��Һ��c��Cu2+��=0.0002mol/L������Ksp����pH��
��� �⣺��1��Խ����ˮ����Σ�Խ����������ӣ���ͬŨ�ȵ�������Һ��pHԽ�����ε�ˮ��̶�Խ����������ˮ�������̼���ƣ�����������������ӵ���������CO32-�����ݱ������ݣ�������ǿ����CH3COOH��Ũ�Ⱦ�Ϊ0.01mol•L-1 ���������������Һ�ֱ�ϡ�� 100����pH�仯������CH3COOH���ʴ�Ϊ��CO32-��CH3COOH��
��2����100ml 0.1mol/L NaHCO3��100ml 0.1mol/L Na2CO3 ������Һ��̼������ӵ�ˮ��̶ȴ���̼��������ӣ�����ˮ��ԭ���������Ӷ�ˮ�ĵ���ƽ��Ĵٽ���������Һ��ˮ�������H+�����Ǣ٣��ڣ��ʴ�Ϊ������
��̼������ӵ�ˮ��̶ȴ���̼��������ӣ�����������Ũ�Ȣ٣��ڣ���Һ�������ӵ����ʵ���Ũ��֮�͵��������Ӻ�������Ũ��֮�ͣ�������Ũ��һ��������������Ũ��Խ����Һ�������ӵ����ʵ���Ũ��֮��Խ�������Ǣ٣��ڣ��ʴ�Ϊ������
��3��̼���������ˮ������̼����Ӻ����������ӣ�ˮ�����ӷ���ʽΪ��HCO3-+H2O?H2CO3+OH-�������£�0.1mol•L-1NaHCO3��Һ��pH����8��c��OH-����c��H+����˵��HCO3-ˮ��̶ȴ��������̶ȣ���c��Na+����c��HCO3-����c��H2CO3����c��CO32-����ˮ��̶Ȳ�������c��HCO3-����c��H2CO3����c��HCO3-����c��OH-������Һ����������Դ��ˮ�ĵ�����HCO3-ˮ�⣬��c��OH-����c��H2CO3��������c��Na+����c��HCO3-����c��OH-����c��H2CO3����c��CO32-����
�ʴ�Ϊ��HCO3-+H2O?H2CO3+OH-��c��Na+����c��HCO3-����c��OH-����c��H2CO3����c��CO32-����
��4����300mL 1mol•L-1��NaOH�����ʵ���=1mol/L��0.3L=0.3mol����״����4.48LCO2�����ʵ���=$\frac{4.48L}{22.4L/mol}$=0.2mol�������������ƺͶ�����̼��Ӧ����ʽΪ2CO2+3NaOH=Na2CO3+NaHCO3+H2O����Һ��̼�������ˮ���ʹ��Һ�ʼ��ԣ���c��OH-����c��H+����̼�������ˮ������̼��������ӣ�����c��HCO3-����c��CO32-��������ˮ��̶Ƚ�С������c��CO32-����c��OH-��������Һ�и�����Ũ�ȴ�С˳����c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+�����ʴ�Ϊ��c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
��pHС�������ȳ������ܶȻ�����ԽС������Խ�ȳ���������Cu2+�ȳ�����KSP[��Fe��OH��2]��KSP[��Mg��OH��2]��0.2mol/L��CuSO4��Һ��Cu2+������Ϊ��ȫ��ʹCu2+Ũ�Ƚ���ԭ����ǧ��֮һ����Ӧ����Һ���NaOH��Һ��c��Cu2+��=0.0002mol/L��c��0H-��=$\sqrt{\frac{2��1{0}^{-20}}{0.0002}}$=10-8mol/L��c��H+��=10-6mol/L������pH=6���ʴ�Ϊ��Cu2+������6��
���� ���⿼����������ʵĵ��롢�ε�ˮ�⡢����Ũ�ȵıȽϡ�Ksp��Ӧ�õ�֪ʶ�㣬��Ŀ�����֪ʶ��϶࣬��Ŀ�Ѷ��еȣ�ע�������Һ������Ũ�ȴ�С�Ƚϵķ�������Ҫѧ��������ʵ�Ļ���֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | AlCl3 | B�� | KCl | C�� | CaCl2 | D�� | LiCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Һ | B�� | ���� | ||
| C�� | ����Һ | D�� | �������ݵ�����Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
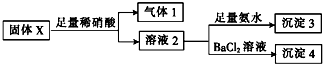
| A�� | ����1һ����NO���ܺ�CO2 | |
| B�� | ����3һ����Mg��OH��2һ������Al��OH��3 | |
| C�� | ����4����ΪBaCO3��BaSO3�������� | |
| D�� | �����ĩX��һ����MgCl2��Na2SO3��������KAlO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��ʯī�缫���200mL CuSO4��Һ���������е���ת�����ʵ���n��e-��������������V ��g������״�����Ĺ�ϵ��ͼ��ʾ������˵���У���ȷ���ǣ�������
��ʯī�缫���200mL CuSO4��Һ���������е���ת�����ʵ���n��e-��������������V ��g������״�����Ĺ�ϵ��ͼ��ʾ������˵���У���ȷ���ǣ�������| A�� | ���ǰCuSO4��Һ�����ʵ���Ũ��Ϊ2mol/L | |
| B�� | ����������Һ��c��H+��=2mol/L | |
| C�� | ��n��e-��=0.6molʱ��V��H2����V��O2��=2��3 | |
| D�� | ��������Һ�м���16gCuO������Һ�ɻָ�Ϊ���ǰ��Ũ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȫ����56g Fe��Ҫ��������33.6 L | |
| B�� | 7.8g Na2O2����100mlˮ��ת�Ƶ�����Ϊ0.1NA | |
| C�� | ��100mL 1mol/L��NaHSO3��Һ�м�������������������Ӧ������������0.4mol | |
| D�� | �������ȷ�Ӧ��������ԭ�õ�16.8g�����ʣ���Ӧ��Fe�õ���0.9NA���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����
���� ��
��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com