
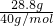 =0.72mol��������Ԫ���غ��֪����Һ��n��Na2CO3��=
=0.72mol��������Ԫ���غ��֪����Һ��n��Na2CO3��=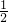 n��NaOH��=0.36mol����c��Na2CO3��=
n��NaOH��=0.36mol����c��Na2CO3��=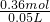 =7.2 mol/L��
=7.2 mol/L��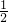 n��NaOH�����ٸ���c=
n��NaOH�����ٸ���c= ���㣮
���㣮

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
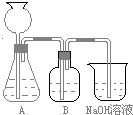
(10��)ij��γ�С������50 mLNaOH��Һ����CO2���壬�Ʊ�Na2CO3��Һ��Ϊ�˷�ֹͨ�������CO2��������NaHCO3�����������ʵ�鲽�裺
a��ȡ25 mL NaOH��Һ���չ�����CO2���壬��CO2���岻���ܽ⣻
b��С�������Һ1��2 min�������ܽ�����Һ�е�CO2���壻
c���ڵõ�����Һ�м�����һ��(25 mL)NaOH��Һ��ʹ���ֻ�Ϸ�Ӧ��
(1)�˷������Ƶýϴ�����Na2CO3��д��c��������ӷ���ʽ_________________��
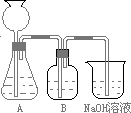
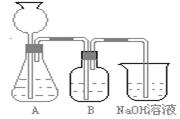
�˷�����һ����ʵ��װ������ͼ��ʾ��
(2)���뷴Ӧ��ǰ����μ�������װ�õ������ԣ�________________________ ________________________________________________
(3)���ô���ʯ��������CO2����װ��B��ʢ�ŵ��Լ���_____ _��������_____________________________________________
(4)��ʵ����ͨ���Ʒ��У�װ��A������Ϊ����______ (�����)����ķ���װ�ã�
��HCl����H2����O2����NH3
(5)��֪����NaOH��Һ�����ʵ���������Ϊ40%�������¸���Һ�ܶ�Ϊ1.44 g/mL�����跴Ӧǰ����Һ��������䣬������ʵ���������ô��ַ����Ʊ�����Na2CO3��Һ�����ʵ���Ũ��Ϊ_______ mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
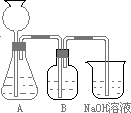
��18�֣�ij��γ�С������50mLNaOH��Һ����CO2���壬�Ʊ�Na2CO3��Һ��Ϊ�˷�ֹͨ���CO2�����������NaHCO3�����������ʵ�鲽�裺
a.ȡ25 mL NaOH��Һ���չ�����CO2���壬��CO2���岻���ܽ⣻
b.С�������Һ1��2 min��
c.�ڵõ�����Һ�м�����һ��(25mL)NaOH��Һ��ʹ���ֻ�Ϸ�Ӧ��
 (1)�˷������Ƶýϴ�����Na2CO3��д��a��c�����Ļ�ѧ��Ӧ����____________________��____________________________��
(1)�˷������Ƶýϴ�����Na2CO3��д��a��c�����Ļ�ѧ��Ӧ����____________________��____________________________��
�˷�����һ����ʵ��װ������ͼ��ʾ��
(2)���뷴Ӧ��ǰ����μ������װ�õ�������______________________________________��
(3)װ��B��ʢ�ŵ��Լ���______________��
������___________________ ____________��
(4)��ʵ����ͨ���Ʒ��У�װ��A������Ϊ����_____________ ����ķ���װ��(�����)��
��CH2==CH2 ��H2S ��CH4 ��CH��CH ��H2
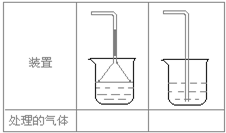
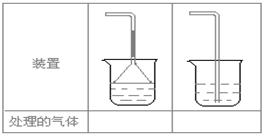
(5)ʵ������ȡ�������壺��NH3����Cl2����HCl����H2S����CH4�� ��CO����CO2����O2ʱ�����ڱ������β����������������ͼ��ʾװ�ý��д����ģ���������������װ��ͼ���·��ո��ڡ�
(6)��֪����NaOH��Һ�����ʵ���������Ϊ40%�������¸���Һ�ܶ�Ϊ1.44 g / mL�����跴Ӧǰ����Һ��������䣬������ʵ���������ô��ַ����Ʊ�����Na2CO3��Һ�����ʵ���Ũ��Ϊ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ɽ��ʡ�����и�һ��ѧ������������⻯ѧ�Ծ� ���ͣ�ʵ����
(10��)ij��γ�С������50 mL NaOH��Һ����CO2���壬�Ʊ�Na2CO3��Һ��Ϊ�˷�ֹͨ�������CO2��������NaHCO3�����������ʵ�鲽�裺
a��ȡ25 mL NaOH��Һ���չ�����CO2���壬��CO2���岻���ܽ⣻
b��С�������Һ1��2 min�������ܽ�����Һ�е�CO2���壻
c���ڵõ�����Һ�м�����һ��(25 mL)NaOH��Һ��ʹ���ֻ�Ϸ�Ӧ��
(1)�˷������Ƶýϴ�����Na2CO3��д��c��������ӷ���ʽ_________________��
�˷�����һ����ʵ��װ������ͼ��ʾ��

(2)���뷴Ӧ��ǰ����μ�������װ�õ������ԣ�________________________ ________________________________________________
(3)���ô���ʯ��������CO2����װ��B��ʢ�ŵ��Լ���_____ _��������_____________________________________________
(4)��ʵ����ͨ���Ʒ��У�װ��A������Ϊ����______ (�����)����ķ���װ�ã�
��HCl����H2����O2����NH3
(5)��֪����NaOH��Һ�����ʵ���������Ϊ40%�������¸���Һ�ܶ�Ϊ1.44 g/mL�����跴Ӧǰ����Һ��������䣬������ʵ���������ô��ַ����Ʊ�����Na2CO3��Һ�����ʵ���Ũ��Ϊ_______ mol/L��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com